Research Article
Changes in Serum Markers of Atherogenesis and Hematological Profile after the consumption of Quail eggs

Ekpenyong CE*, Patrick ES and Akpan KS
Department of Physiology, Faculty of Basic Medical Sciences, University of Uyo, Uyo, Nigeria
*Address for Correspondence: Dr. Christopher E. Ekpenyong, Department of Physiology, Faculty of Basic Medical Sciences, University of Uyo, AkwaIbom State, Nigeria, Tel: +2348023347719, Email: [email protected]; [email protected]
Dates: Submitted: 14 April 2017; Approved: 08 May 2017; Published: 10 May 2017
How to cite this article: Ekpenyong CE, Patrick ES, Akpan KS. Changes in Serum Markers of Atherogenesis and Hematological Profile after the consumption of Quail eggs. Arch Food Nutr Sci. 2017; 1: 001-011.
DOI: 10.29328/journal.afns.1001001
Copyright License: © 2017 Ekpenyong CE, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
Previous studies suggest that diets with more eggs than is recommended may be used as part of a healthy diet in some countries. However, whether quail egg diets could form part of such diet has not been explored. The aim of the present study is to evaluate the effect of quail egg consumption on serum markers of atherogenesis and hematological parameters in healthy volunteers. Thirty adult subjects participated in this study after ascertaining their baseline health status. They were fed 3 eggs per day and 8hourlyfor 30 days. They were evaluated for serum levels of cholesterol sub-fractions, AIP and hematological parameters at days 0, 10 and 30 after the consumption of quail eggs. At day10, serum levels of cholesterol sub-fractions (TG, HDL-C and LDL-C) were not significantly (p>0.05) different from the corresponding values at baseline. Serum levels of VLDL-C and calculated AIP significantly (p<0.05) decreased compared to the levels at baseline. At day 30, serum levels of HDL-C, TG and VLDL-C significantly (p<0.05) increased, while LDL-C and AIP significantly decreased. Also, total RBC, HB, PCV, MCV, MCH and MCHC were not significantly different from the levels at baseline. At day 30, RBC, PCV and HB significantly (p<0.05) increased compared to the levels at baseline, while MCV, MCH and MCHC were not significantly (p>0.05) different from the baseline values.
Indeed, long-term consumption of quail egg may be associated with improvement in serum markers of atherogenesis and hematological parameters due to its varied nutrient constituents and their activities.
INTRODUCTION
The rising incidence of cardiovascular diseases (CVDs) constitutes an enormous public health concern because of its association with increased morbidity and mortality burdens. CVDs including coronary heart disease (CHD) are responsible for one-third of all deaths in the world with the projection that by 2030, >2.3 million people will be affected by CVDs. [1,2].
The etiology of CVD is controversial and speculative and may involve interactions between socio-cultural, genetics, environmental and lifestyle factors including diets [3]. From the dietary perspective, it is known that diet constitutes a modifiable risk factor for the initiation, progression and endpoints of CVDs [4]. Available data indicate that diet high in saturated fatty acids and cholesterol, but low in fibers, plant foods, and unsaturated fatty acids is detrimental to cardiovascular health. However, studies on the effect of specific food types on CVDs risk are yet to receive global attention.
Quail eggs (Coturnix coturnix) diet is one such diet which, although extensively consumed by humans, has not been adequately explored for its cardio-protective/or cardio-toxic effect. Literature evidence indicates that quail egg is a rich source of many nutrients including carbohydrates, ash, vitamins (vitamins A, B (B1, B2, niacin, B6, biotin, folic and panthotenate acid), C, D and E), trace elements and minerals (e.g., magnesium, zinc, selenium, phosphorus and iron), proteins, essential fatty acid (omega-3 and omega-6 fatty acids), essential amino acids (leucin) and non-essential amino acids (oleic ) [5]. Interestingly, these nutrients are present in higher concentrations than are found in other types of eggs. Quail eggs, unlike others, is claimed to have the capacity to strengthen the immune system, protect the central nervous system, and is active against anemia, metabolic syndrome clusters and tuberculosis [5-7]. This is likely due to its anti-oxidative, anti-inflammatory and immune-modulatory activities [8].
Consequent upon the foregoing, public awareness of the nutritional and health benefits of quail eggs has increased the interest in its consumption by the general population For instance, in some countries people consume quail eggs for its action against anemia, asthma, diabetes mellitus, ulcers, tuberculosis, hypertension and arteriosclerosis [9]. In Nigeria, anecdotal evidence indicates that many people consumed quail eggs for cardio-protective effect. However, this increased interest in the consumption of quail eggs by the general population without empirical data justifying the claimed biological activities including the atherosclerosis cardiovascular protective effects prompted the present study. Few available studies were animal based and cannot be completely applicable to humans. Also, these studies were mainly secondary preventive studies [10,11].
Therefore, the aim of the present study is to accurately evaluate the effect of quail consumption on serum markers of atherogenesis and hematological profile in healthy human volunteers.
METHODS
Thirty adult participants (16 males and 14 females) ages between 18 and 35 years were included in this study. Out of the 45 adults who were initially invited to participate, 15 were excluded for not meeting the inclusion criteria. Prior to the commencement of the study, all participants underwent a pre-survey medical screening performed by a medical doctor so as to ascertain the baseline values of the assessed indices and their general health status. The pre-survey screening included clinical screening, evaluation of renal function indices (serum creatinine, acid-base status, electrolyte levels (sodium, potassium, chloride and calcium), liver function (serum transaminases, protein (total and sub-fractions), bilirubin (total and conjugated and hematological indices.
Baseline urinalysis was also carried out. Weight (kg), height (m), body mass index (kg/m2) pulse rate (PR), blood pressure (BP) mmHg and respiratory rate (RR) were measured. Participants with abnormal value of any of the aforementioned tests were excluded from the study. Other exclusion criteria included decline participation, missing information, history of liver or kidney disease, evidence of any metabolic syndrome clusters e.g., hypertension, diabetes mellitus, dyslipidemia and obesity.
Smokers, alcohol users, caffeine users and those on medications for treatment of metabolic syndrome clusters or contraceptives were excluded. Others are those known to be allergic to quail eggs or any of their constituents. Informed written consent was obtained from all participants and the study protocol was approved by the Institutional Research and Ethics Committee at the University of Uyo, Nigeria. The study was conducted in 2015 in line with the rules set forth in the Declaration of Helsinki governing the use of human subjects.
As a precautionary measure to avoid the confounding effect of other diets, participants were instructed to remain on their regular diet but should avoid high cholesterol and protein diets (such as meat), excessive physical exertion and other forms of egg diet.
Fresh quail eggs were obtained from an agricultural farm in Uyo Metropolis, Akwa Ibom State. They were properly identified and stored at room temperature to maintain quality. Each participant was fed with one boiled quail egg 8 hourly for the period of 30 days. This dose range was adapted from a previous human study in which participants exhibited no obvious clinical or biochemical evidence of toxicities [12]. Nevertheless, tolerability evaluation was performed using 5 subjects who were given 3 eggs per 1 day for 1 week and were thereafter evaluated for clinical or biochemical evidence toxicities. Physical examinations were performed on each participant to check evidence of adverse reaction. Participants were closely monitored and were instructed to report any unusual symptoms during this period.
Sample collection and processing
At days 0, 10 and 30 fasting venous blood samples were collected from participants into sample bottles containing dipotassiumethylenediaminetera acetate (K2EDTA) and without K2EDTA for use in evaluating hematological induces and lipid profile respectively.
Assessment measure
Three surveys instrument were used for this study. These include a semi-structured questionnaire adapted from previous studies on health effect of egg consumption (Techakriengkrai et al 2012). Serum lipid sub-fractions including total cholesterol (T-chol), high density lipoprotein (HDL), low density lipoprotein (LDL), very low density lipoprotein (VLDL) and triglyceride (TG) were measured with a multi-channel automated system (Lipid pro TM, Model KM-001A) :Info pia Co. Ltd. South Korea.
The hematological indices were determined using the SYSMEX Kx-21 automated hematology analyzer (Kobe, Japan). The indices measured included total red blood cell count (RBC), packed cell volume (PCV), hemoglobin concentration (HB), mean cell volume (MCV), mean cell hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), total white blood cells (WBC) and white blood cell lineages (monocytes (MONO), lymphocytes (LYM), neutrophils (NEU), eosinophils (EOS) and basophiles (BAS) and platelet counts (PL)).
Estimation of Atherogenic Index of Plasma (AIP)
This is a mathematical relationship between cholesterol sub-fractions (LDL-C, HDL-C, TG, and TC), and is expressed as follows:
HDL-C/TC, LDL-C/HDL and TG/HDL-C.
These ratios predict the likelihood of atherosclerosis development. Out of the aforementioned ratios, log (TG/HDL-C) is the most accurate and useful in atherosclerosis risk determination. AIP of 0.03-0.1 indicates low risk, 0.1 to 0.24 indicates medium risk while AIP>0.24 suggests high CVD risk [10,13,14].
Statistical analysis
Data obtained were expressed as mean±standard deviation (Mean±SD). Comparison between groups was performed using one way analysis of variance (ANOVA). This was followed by Ducan Multiple Range Test (DMRT) to determine significant differences between groups. Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) version 20.0 and Graphipad Prism 5.0. Differences were considered statistically significant at p<0.05.
RESULTS
The socio-demographic characteristics of the study subjects at baseline showed that they were all Christians, black, of Ibibio ethnicity and with a mean age of 27.4±0.30. Also, a greater number of them (53.7%) were males. The mean weight, height, pulse pressure, SBP, DBP and respiratory rate were 60.74±1.93kg, 1.62±0.01kg, 45.87±1.04, 120.53±1.89 mmHg, 74.64±1.62mmHg and 18.56±1.52/min respectively (Table 1).
| Table 1: Baseline demographic characteristics of study participants. | |
| Variables | Baseline values |
| Demographic Characteristics Average age Sex Male Female Race (% black) Ethnic (% Ibibio) Religion (% Christianity) |
27.42±0.30
16 (53.3) 14 (46.7) 30 (100) 30 (100) 30 (100) |
| Clinical Characteristics Weight (kg) Height (m2) Pulse pressure(PP) Systolic Blood Pressure(BP) (mmHg) Diastolic Blood Pressure(BP) (mmHg) Respiratory rate (RR) counts/min. |
60.74±1.93 1.62±0.01 45.87±1.04 120.53±1.89 74.64±1.62 18.56±1.52 |
| Values in parenthesis are expressed in percentages | |
Table 2 shows that at day 10, RBC, HB, PCV, MCV, MCH and MCHC were not significantly different from the baseline values in both male and female subjects. At day 30, RBC, PCV and HB significantly increased in both male and female subjects (p<0.05), whereas MCV, MCH and MCHC were not significantly different from the corresponding values at baseline.
| Table 2: Acute and chronic effects of quail egg consumption on red blood cell indices in male and female subjects. | ||||||
| Parameters | Male | Female | ||||
| Baseline | Day 10 | Day 30 | Baseline | Day 10 | Day 30 | |
| RBC (106/µL) | 5.08±0.48a | 5.15±0.45a | 5.47±0.41b | 4.54±0.43a | 4.59±0.51a | 5.04±1.00b |
| HB(g/dl) | 14.01±1.08a | 14.62±1.04a | 15.12±0.91b | 12.01±1.01a | 12.39±1.11a | 13.65±1.08b |
| PCV (%) | 42.03±2.87a | 43.86±2.70a | 45.36±2.40b | 36.03±2.48a | 37.17±2.60a | 40.95±3.06a |
| MCV (fl) | 86.27±4.29a | 89.73±8.63a | 86.71±6.33a | 84.49±5.51a | 80.77±21.41a | 83.18±11.69a |
| MCH (pg) | 27.16±1.82a | 28.63±2.78a | 27.99±2.54a | 25.84±2.49a | 27.19±3.18a | 27.76±4.11a |
| MCHC (g/dl) | 31.51±1.35a | 31.90±1.33a | 32.14±1.21a | 30.65±1.35a | 31.93±2.32a | 33.42±1.49a |
| Values are reported as mean±SEM a=significantly different from Baseline value (p<0.05) b=significantly different from 10 days value (p<0.05) RBC= red blood cell counts, HB= hemoglobin concentration, PCV=packed cell volume, MCV= mean cell volume, MCH= mean cell hemoglobin, MCHC= mean cell hemoglobin concentration |
||||||
Table 3 shows that at day 10, PL, WBC, and LYM counts were not significantly different from baseline values in both male and female subjects (p>0.05).
| Table 3: Acute and chronic effects of quail egg consumption on white blood cell indices in male and female subjects. | ||||||
| Parameters | Male | Female | ||||
| Baseline | Day 10 | Day 30 | Baseline | Day 10 | Day 30 | |
| PL (103/µL) | 175.93±55.05a | 178.07±41.50a | 171.87±55.81a | 239.67±24.03a | 247.07±36.68a | 243.80±20.27a |
| WBC (103/µL) | 4.65±0.90a | 4.97±1.43a | 5.81±1.50b | 5.35±1.33a | 5.37±1.22a | 6.45±2.58a |
| LYM (%) | 44.92±7.06a | 44.39±8.00a | 44.52±8.10a | 43.63±4.74a | 43.25±6.92a | 42.23±5.98a |
| MONO (%) | 8.72±1.09a | 12.75±1.95b | 10.35±1.51b | 7.96±1.38a | 12.44±2.37b | 10.85±1.02b |
| NEU (%) | 46.36±2.48a | 44.95±2.41a | 47.31±2.52a | 44.17±3.79a | 46.75±2.30b | 49.47±4.55b |
| Values are reported as mean±SEM. a=significantly different from Baseline value (p<0.05). b=significantly different from 10 days value (p<0.05). PL=platelet counts, WBC= white blood cell counts, LYM= lymphocyte counts, MONO=monocyte counts, NEU=neutrophil counts. |
||||||
Additionally, MONO counts were significantly higher than the correspondent value at baseline, while NEU counts were not significantly different from the baseline values in male subjects. Both NEU and MONO counts significantly increased (p <0.05) in female subjects.
At day 30, PL, LYM and NEU counts were not significantly different from baseline values (p>0.05) whereas total WBC, and MONO counts significantly increased in male subjects. In female subjects, PL, WBC, and LYM counts were not significantly differently from baseline values whereas MONO and NEU counts significantly increased (p<0.05) compared to the corresponding values at baseline.
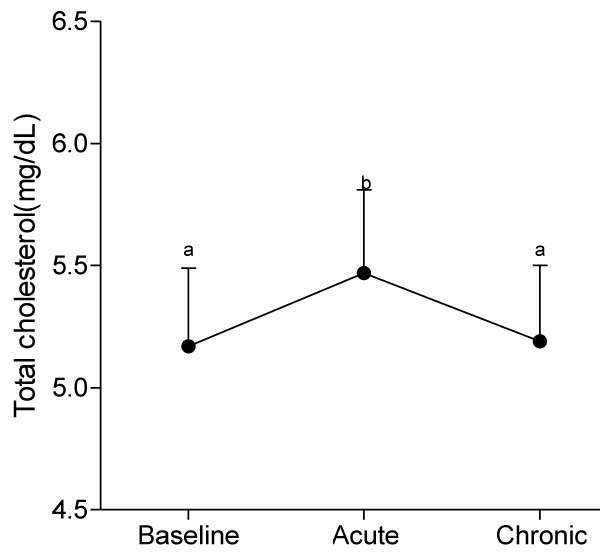
Evaluation of the serum lipid sub-fractions showed that at day 10, consumption of quail eggs caused a significant increase (p<0.05) in serum T-chol of participants, and by day 30 serum level of T-chol was not significantly different from baseline value (p>0.05).
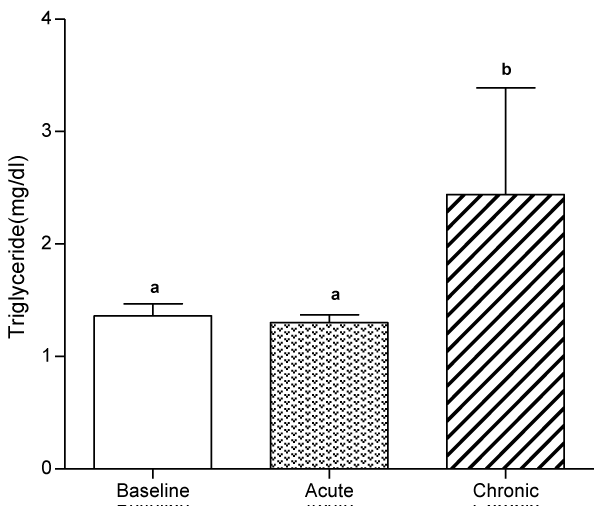
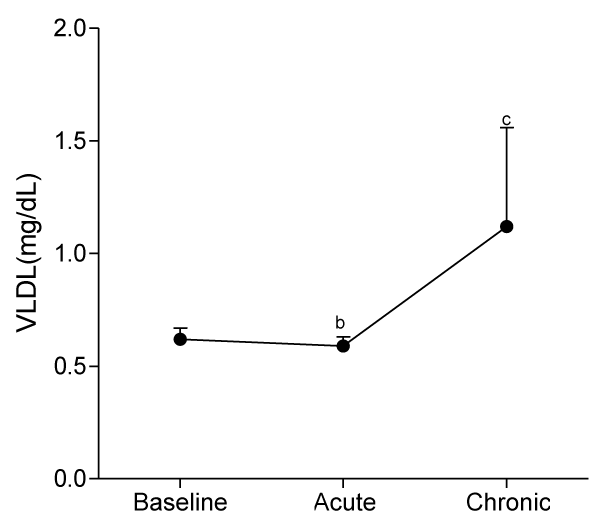
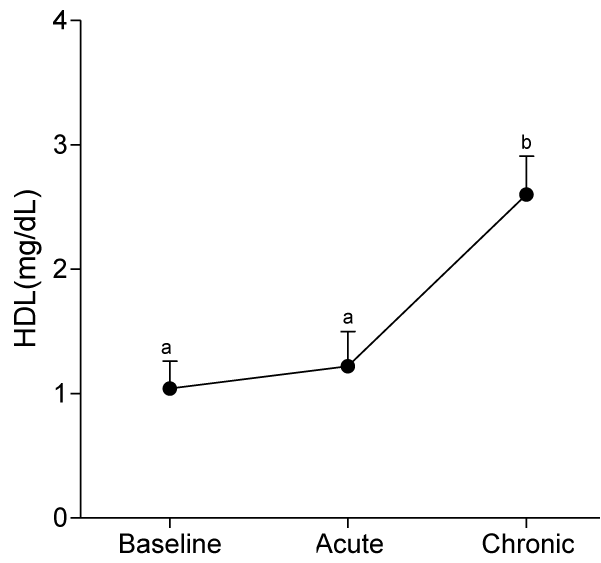
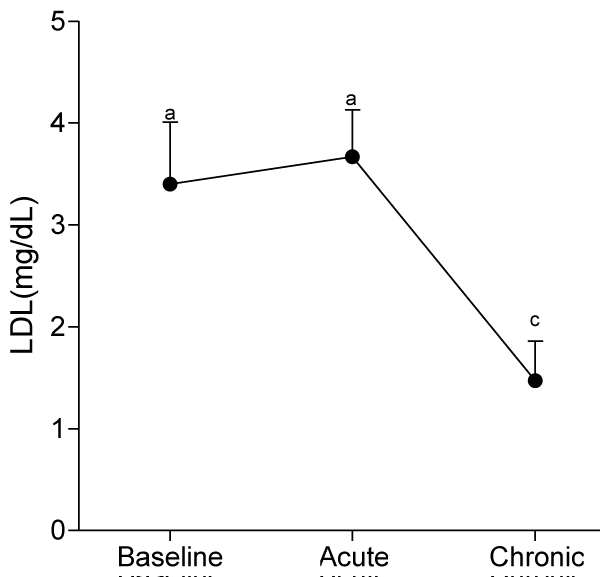
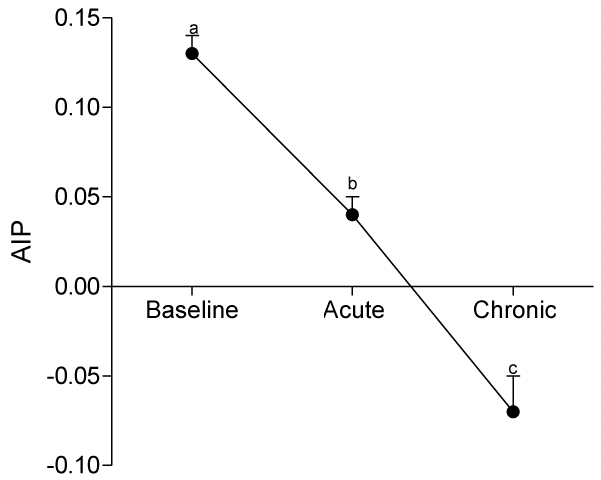
At day 10, serum levels of TG, HDL-C and LDL-C were not significantly different from baseline values, whereas serum levels of VLDL-C and AIP significantly decreased (p<0.05) compared to baseline values.
At day 30, ingestion of quail eggs caused significant increase in serum levels of HDL-C, TG, and VLDL-C and decrease in serum level of LDL-C and AIP (Figures 1-6).
DISCUSSION
In the present study, we found out that long-term consumption of quail eggs diet caused significant changes in serum levels of cholesterol sub-fractions including a 57% decrease in serum level of LDL-C and 150% and 79% increases in serum levels of HDL-C and TG respectively. Also, AIP decreased by 69% and 46% on short-term and long-term consumption of quail eggs respectively as compared to the corresponding values at baseline. These results suggest that quail eggs diet has the potential to decrease the risk of atherosclerosis by significantly decreasing serum level of LDL-C (Bad cholesterol), and simultaneously increasing serum levels of HDL-C (good cholesterol) especially on prolonged consumption.
Although many investigators have identified several factors promoting atherosclerosis including low level of serum HDL-C and high levels of serum LDL-C and total cholesterol, and hyper-triglyceridemia, oxidative modification of native LDL-C is central to the pathophysiology of atherosclerosis due the ability of the oxidized LDL-C to initiate/drive processes leading to atherosclerosis such as increased production of biologically active compounds and increased expression of endothelial cell surface molecules. There is also recruitment of circulating inflammatory cells and transformation of monocytes into macrophages and foam cells.
Also, oxidized LDL-C is cytotoxic and can cause injury to the endothelial and smooth muscle cells, leading to damage of the arterial wall [15], increased intimal proliferation, fibrosis, plaque formation, slow chronic inflammatory reaction and atherosclerosis. There could also be plaque rapture and associated thrombus formation.
These negative impacts on vascular endothelial function can impair nitric oxide synthesis and through series of events (such as vasoconstriction, increase thromocyte aggregation, monocyte migration to vascular wall, and increased formation of foam cells) lead to atherosclerosis. The slow chronic inflammatory reactions could lead to increase production of inflammatory cytokines such as interleukin (IL)-IB, IL-6, tumor necrosis factor-alpha (TNF-α), and high sensitivity C-reactive protein (hs-CRP), endothelial dysfunction and atherosclerosis, [16].
The observed changes in serum cholesterol sub-fractions (increased HDL-C and decreased LDL-C and AIP) and haematological parameters following the consumption of quail eggs diet is partly attributable to its rich natural nutrient constituents including vitamins (vitamins A, B (B1, B2, niacin, B6, biotin and folic acid), C, D and E), trace elements and minerals (Mg2+, Zn, Se, Po4 and Fe) protein, polyunsaturated fats and essential and non-essential amino acids [5]. For instance, quail eggs contain more polyunsaturated fatty acid (PUFA) than saturated fatty acid (SFA) and trans-fatty acid [5].
PUFA is known to reduce plasma LDL-C concentration and hence atherosclerosis risk, by increasing LDL-C receptor activity, reducing the production of LDL-C and increasing receptor dependent LDL-C transport and hence plasma concentration of LDL-C [17].
Several studies including the Lipid Research Clinic Coronary Primary Prevention Trial [18], Framingham study [19] and other clinical and epidemiologic studies have indicated that reducing LDL-C, reduces atherosclerosis and CVD risk. Likewise other studies including the Homozygous Familial Hypercholesteremia showed that elevated LDL-C is the single most important factor driving atherogenesis and can act alone to promote atherosclerosis. However, it is noteworthy that oxidation of LDL-C as a critical step in atherosclerosis is promoted by several factors including oxidative stress, inflammatory reaction and immune system dysfunction. For instance, the production of superoxide ions following oxidative stress, cellular release of lipo-oxygenase and other cellular enzymes following inflammation, deficiency of antioxidant vitamins, type of fatty acids and the size of LDL-C particles [15] are known triggers of LDL-C oxidation. Therefore, the anti-atherosclerosis effect of quail eggs diet is partly due to the presence, and activities of their anti-oxidative, anti-inflammatory and immune modulatory nutrient constituents. Some of these nutrients can act either alone or in combination to reduce the size of LDL-C, decrease serum levels of LDL-C and increase resistance of LDL-C to oxidation, while increasing serum levels of HDL-C and haematological indices as observed in the present study.
One cross-sectional study. [5], to evaluate nutrient constituents of quail eggs detected high levels of vitamins (vitamins A, D and E), minerals (iron and zinc) and sex hormones. Vitamin A exhibits its anti-atherosclerotic effect by reducing oxidation of LDL-C in arteries, quenching of singlet oxygen [20] and inducing the formation of regulatory T-cells (Tregs) through its anti-inflammatory and immune modulatory effects [16].
Likewise, vitamin D protects against atherosclerosis through its anti-oxidative, anti- inflammatory and immune function modulation. Also, the action of vitamin D against some metabolic aberrations such as diabetes mellitus, hypertension and dyslipidemia is known to secondarily protect against atherosclerosis and CVD risk [21]. Vitamin E prevents oxidation of LDL-C. Zn modulates serum total lipid, cholesterol, TG, LDL-C and HDL-C. Deficiency of Zn leads to abnormalities in serum levels of the aforementioned lipid sub-fractions and atherosclerosis [22].
The rich nutrient constituents of quail eggs diet provides explanation for the improvement in hematological parameters of the participants including significant increases in RBC, PCV and HB concentrations at day 30 of the present study. According to Tunsaringkarn et al., quail eggs diet is rich in fats [5], carbohydrate, proteins, vitamins and minerals. Interestingly, these nutrients are present in higher concentrations (about 3-4 times) than chicken eggs. They possess hematopoiesis boosting effects, and are used traditionally in the management of anemia [5,23]. Indeed, the presence of these nutrients in quail eggs could have improved their total plasma concentrations in the participants and served as precursors for hematopoiesis [24], thereby causing the observed improvement in hematological parameters.
Intriguiningly, we found out a significant reduction in the risk of atherosclerosis at day 30, despite the significant increases in serum levels of TG and VLDL. This indicates the significant and independent role of abnormal serum levels of HDL-C and LDL-C in atherosclerosis, and confirmed previous studies with similar findings [25-27]. The actions of HDL-C and LDL-C in atherosclerosis are antagonistic, while LDL-C promotes atherosclerosis, HDL-C protects against atherosclerosis [28]. Therefore, the significant increase serum level of HDL-C and decrease serum level of LDL-C observed in the present study is beneficial to cardiovascular health.
Our results are at variance with previous studies [10,11] which showed negative anti-atherogenic effect of quail egg diets. Absence of effect in these studies as against the present study may be due to differences in methodological approach. Some of the studies with negative effects were performed in models of experimentally-induced dyslipidemia [10,11] whereas the present study was conducted in healthy subjects with baseline normal serum lipid profile.
The finding of positive anti-atherosclerosis effect of quail egg consumption in the present study suggests that quail egg diets and their constituents maybe more effective in preventing the initiation/onset of dyslipidemia, but ineffective in already established dyslipidemia. The discrepant findings could also be due to individual variability and genetic susceptibility to LDLs oxidation [29]. Previous studies have shown that some of the major constituents of quail egg diet (such as vitamin E) have greater protection against the onset of primary atherosclerotic lesions than pre-existing/already established atherosclerotic lesions or in individuals with a high coronary heart disease risk background such as individuals with metabolic aberration clusters (e.g., diabetes mellitus, hypertension and obesity) which are common features of most clinical trials and secondary prevention studies [16,30].
Serum levels of TG and VLDL were not significantly different from baseline values at day 10. However, significant increases were obtained at day 30, suggesting the absence of effect of quail constituents on TG and VLDL metabolism on a short-term consumption, and in particular, the activation of endothelium bound lipoprotein lipase which hydrolyses tri-acylglycerol into fatty acid [31]. This observation obtains additional support from a previous study with similar findings [10].
Some limitations of the present study should be considered in the interpretation of the results. First, we could not analyze the actual nutrient constituents of the quail eggs used in the present study. Secondly, the age group of the studied participants limits generalization of the study’s results. Finally, the confounding effects of other un-indentified covariates could have affected results.
CONCLUSION
Long-term consumption of quail eggs may be effective in preventing the initiation/onset of dyslipidemia and hence onset of primary atherosclerotic cardiovascular disease. However, larger studies are warranted to provide further evidence of this promising and inexpensive dietary regimen for prevention of atherosclerosis cardiovascular diseases.
ACKNOWLEDGEMENT
The authors gratefully acknowledge Mr. Samon A. Oyebadejo of Derindam Research Institute of Bio-technology, Uyo, Akwa Ibom State, Nigeria, for his professional assistance.
REFERENCES
- Voutilainen S, Nurmi T, Mursu J, Rissanen TH. Carotenoids and Cardiovascular health. Am J Clin Nutr. 2006; 83: 1265-1271. Ref.: https://goo.gl/il0F51
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006; 3: e442. Ref.: https://goo.gl/YHtK2i
- Stamler J. Established major coronary heart disease epidemiology: from Aetiology to Public Health, Edited by M. Marmot & P. Elliot, New York. Oxford University Press. 1993; 35-66. Ref.: https://goo.gl/m6dO80
- Singh RB, Niaz MA, Ghosh S. Hyperlipidemic and antioxidant effects of Commiphora mukul as an adjunct to dietary therapy in patients with hypercholesterolemia. Cardiovasc Drugs Ther. 1993; 8: 659-664. Ref.: https://goo.gl/XBFbGo
- Tunsaringkarn T, Tungjaroenchai W, Sirwong W. Nutrient benefits of quail (coturnix japonica) eggs. Int J Sci Res Pub. 2013; 3: 1-8. Ref.: https://goo.gl/AHgm2p
- Dobrass F. Quail eggs farming in Nigeria. 2012.
- Oguwike FN, Ebede S, Offor CC, Olisah MC. Activities of quail eggs in female anemic hypertensive subjects in Ogwa Imo State, Nigeria. IOSR J Dental Med Sci. 2014; 13: 106-109. Ref.: https://goo.gl/t6VqgI
- Lalwani P. Quail egg nutrition. 2017. Ref.: https://goo.gl/J4pmYJ
- Anca T, Vacaru-opris I, Teusan V. Aspects regarding some morphological values of the domestic quail eggs (Coturix japonica). Lucr Stiint Zooteh Biotech. 2008; 41: 709-716. Ref.: https://goo.gl/V1ZYTz
- Adeniyi OT. Effect of Japanese quail egg (coturnix Japonica) egg supplementation on poloxamer 407-induced hyperlipidaemic Wister rats. An MSC Thesis. 2014. Ref.: https://goo.gl/ZXxHEi
- Lontchi-Yimagou E, Tanya A, Tchankon C, Ngondi J, Oben J. Metabolic effect of quail eggs in diabetes-induced rats: comparison with chicken eggs. Food Nutr Res. 2016; 60. Ref.: https://goo.gl/vK0j8N
- Techakriengkrai T, Klangjareonchai T, Pakpeankitwattan V, Sritara P, Roongpisuthipong C. The effect of ingestion of eggs and low density lipoprotein (LDL) oxidation on serum lipid profile in hypercholesterolemic women. Songklanakarin J Sci Technol. 2012; 34: 173-178. Ref.: https://goo.gl/Qmz1Wx
- Dobiasova M. AIP-atherogenic index of plasma as a significant predictor of cardiovascular risk: from research to practice. Vnitrni Lekarstvi. 2006; 52: 64-71. Ref.: https://goo.gl/OIfwLi
- Kastelein JJP, van der Steeg WA, Holme I, Gaffney M, Cater NB, et al. Lipids, apolipoproteins and their ratios in relation to cardiovascular events with statin treatment. Circulation. 2008; 117: 3002-3009. Ref.: https://goo.gl/rY4ROi
- Grundy SM. Oxidized LDL and atherogenesis: relation to risk factors for coronary heart disease. Clin Cardiol. 1993; 16: 3-5. Ref.: https://goo.gl/Ipefdn
- Ekpenyong CE. Micronutient vitamin deficiencies and cardiovascular disease risk: advancing current understanding. Eur J Prevent Med. 2017; 5: 1-18. Ref.: https://goo.gl/c7bktg
- Woollet LA, Spady DK, Dietschy JM. Saturated and unsaturated fatty acids independently regulate low density liporpotien receptor activity and production rate. J Lipid Res. 1992; 33: 77-88. Ref.: https://goo.gl/MQBwxt
- The Lipid Research Clinic-Coronary Prevention Trial Results (LRC-CPTR). II. The relationship of reduction of incidence of coronary heart disease. JAMA. 1984. 251: 365-374. Ref.: https://goo.gl/i99ioe
- Kannel WB, Castelli WP, Gordon T. Cholesterol in the production of atherosclerotic disease: New perspectives in the Framingham study. Ann Inter Med. 1979; 90: 85-91. Ref.: https://goo.gl/SmAvaF
- Stahl W, Sies H. Physical quenching of singlet oxygen and cis-trans isomerization of carotinoids. Ann NY Acad Sci. 1993; 691: 10-19. Ref.: https://goo.gl/QeEm2q
- Muscogiuri G, Sorice GP, Ajjan R, Mezza T, Pilza S, et al. Can vitamin D deficiency cause diabetes and cardiovascular disease? Present evidence and future perspectives. Nutr metab Cardiovascular Dis. 2012; 22: 18-27. Ref.: https://goo.gl/AhfMT6
- Ranasinghe P, Wathurapatha WS, Ishara MH, Jayawardana R, Galappatthy P, et al. Effects of zinc supplementation on serum lipids; a systematic review and meta-analysis. Nutr Metab. 2015; 12: 26. Ref.: https://goo.gl/qzRRUz
- Troutman. What are the benefits of quail eggs? 1999-2012. Ref.: https://goo.gl/f0uLfR
- Ekpenyong CE, Daniel NE. Roles of diets and dietary factors in the pathogenesis, management and prevention of abnormal serum uric acid levels. J PharmaNutr. 2014; 3: 29-45. Ref.: https://goo.gl/i8Yz9E
- Gordon T, Castelli WP, Hjortland Mc, et al. High density lipoprotein as a protective factor against coronary heart disease. The Framingham study. Am J Med. 1977; 62: 707-714. Ref.: https://goo.gl/Xa8Pqi
- Wilson PW, Abbott RD, Castelli WP. High density lipoprotein cholelsterol and mortality. The Framingham heart study. Arteriosclerosis. 1988; 8: 737-741. Ref.: https://goo.gl/584kht
- Castelli WP, Garrison RJ, Wilson PWF, et al. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham study. JAMA. 1986; 256: 2835-2838. Ref.: https://goo.gl/fEQsOh
- Barter P. The role of HDL-cholesterol in preventing atherosclerotic disease. European Heart Journal Supplements. 2005; 7: F4-F8. Ref.: https://goo.gl/rGbF7n
- Jailal I, Freeman DA, Grundy SM. Varying susceptibility of different low density lipoprotein to oxidative modification. Arteriosc Thrombosis. 1991; 11: 482-488. Ref.: https://goo.gl/A602ce
- Feki M, Souissi M, Mokhtar E, Hsairi M, Kaabachi N,et al. Vitamin E and Coronary Heart Disease in Tunisians. Clin Chem. 2000; 46: 1401-1405. Ref.: https://goo.gl/UcGWHi
- Sikarwar MS, Patil MB. Antihyperlipidemic effect of ethanolic extraction of Hibiscus rosa Sinensis flowers in hyperlidemic rats. RGUHS J Pharm Sci. 2011; 1: 117-122. Ref.: https://goo.gl/Dm5ozI