More Information
Submitted: December 31, 2020 | Approved: February 02, 2021 | Published: February 03, 2021
How to cite this article: Delshad H, Mirmiran P, Mehran L, Tohidi M, Azizi F. Iodine status and thyroid parameters of pregnant women living in an iodine sufficient area. Arch Food Nutr Sci. 2021; 5: 001-006.
DOI: 10.29328/journal.afns.1001026
Copyright License: © 2021 Delshad H, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Iodine; Thyroid volume; Pregnancy; Urinary iodine; Iran
Iodine status and thyroid parameters of pregnant women living in an iodine sufficient area
Hossein Delshad1, Parvin Mirmiran2, Ladan Mehran1, Maryam Tohidi3 and Fereidoun Azizi1*
1Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, IR Iran
2Nutrition Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, IR Iran
3Prevention of Metabolic Disorders Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti, University of Medical Sciences, Tehran, IR Iran
*Address for Correspondence: Fereidoun Azizi, M.D. Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic of Iran, Email: [email protected]; [email protected]
During the last few decades painstaking efforts have been made to eliminate iodine deficiency throughout the world. Todays in regions where dietary iodine intake is adequate or borderline, the main focus is increasing dietary iodine supply in the target population during pregnancy and the first years of life.
Objective: The aim of this study was to obtain longitudinal data on urinary iodine excretion and the changes of maternal thyroid parameters in two groups of healthy women with mild-to-moderate iodine deficiency and iodine sufficiency residing in an iodine replete area of Tehran capital city of IR Iran, for more than one decade.
Research designs and methods: The present study is part of a cohort study, investigating the relative influences of iodine intake on thyroid size and function of mothers and their infants during and after pregnancy. A total of 500 pregnant women enrolled from two mother-child health care centers and was divided into group I, with median urinary iodine excretion (MUIE) < 150 µg/L, and group II with MUIE ≥ 150 µg/L. Sonographic thyroid volume measurement, urinary iodine excretion and thyroid function tests were measured sequentially in all pregnant women during the three trimesters (T) of pregnancy.
Results: The mean ± SD age of the participants was 25.1 ± 5.1 years. The MUIE in group I and II in the first, second and third trimester were 123 and 250 µg/L, 127 and 166 µg/L, 120 and 150 µg/L, respectively. The MUIE in the third trimester of pregnancy in group I did not differ significantly from the values in the first and second trimesters (p = 0.67), but it did decline significantly in group II (p < 0.001). The median thyroid volume of subjects, in the first, second and third trimesters were 7.8, 8.2 and 8.1 ml in group I and 7.5, 8.0 and 8.4 ml in group II, respectively. No difference in thyroid volume was found between two groups in each of the three trimesters of pregnancy (p > 0.05). The mean (± SD) TSH concentration of subjects in first, second and third trimester was 2.3(± 2.6), 2.1(± 1.8), 2.3(± 1.7) mIU/L in group I and 2.1(± 3.1), 2.1(± 1.8) and 2.0(± 1.3) mIU/L in group II, respectively. The trend of TSH rising in group I was 26.7% and in group II it was 13.3%. The mean TSH value in three trimesters did not differ significantly in either groups (p > 0.05). The mean (± SD) total T4 concentrations of subjects in first, second and third trimesters were 13.2(± 3.4), 13.8(± 3.3), 13.0(± 2.9) µg/dl in group I and 13.1(± 3.2), 13.7(± 2.9), 13.4(± 3.2) µg/dl in group II, respectively. The mean total T4 value in three trimesters did not differ significantly in either groups (p > 0.05). There was no correlation between the thyroid volume and three observed parameters (UIE, total T4 and TSH) during the pregnancy in either groups.
Conclusion: Even in areas with well-established universal salt iodization program, pregnancy could be a risk of having iodine deficiency and systematic dietary fortification needs to be implemented in this vulnerable group.
It is well known that thyroid hormones are essential for the development of the central nervous system (CNS). The lack of thyroid hormones during the critical period of maturation of the CNS can result in morphological, physiological and biochemical abnormalities [1]. Iodine is essential for the production of thyroid hormones. The iodine requirement during pregnancy is increased due to an increase in maternal T4 production to maintain maternal euthyroidism, transfer of thyroid hormones and iodine to the fetus and some increase in renal clearance of iodine [2,3]. During second half of gestation a fraction of the maternal inorganic iodine pool is diverted towards the fetal-placental unit [4].
In severely iodine deficient areas, pregnancy results in marked hypothyroidism and intense thyroid stimulation in both mothers and newborns, but in iodine-sufficient areas, physiological iodine losses are not associated with significant changes in the maternal thyroid economy [5,6]. Although there has been considerable progress in the prevention and control of iodine deficiency disorders (IDD) globally, it is estimated that there are still about 39 million infants born each year unprotected from the risk of cognitive damage from iodine deficiency [7]. Todays in regions where dietary iodine intake is adequate or borderline, the main focus is increasing dietary iodine supply in the target populations during the pregnancy and the first years of life. World Health Organization (WHO)-recommended daily iodine requirement are 250 µg for pregnant and lactating women. Although this recommended daily iodine intake can be readily achieved in countries with adequate household consumption of iodine, dietary supplements would be required in other parts of the world for these target groups [8].
A WHO technical consultation on prevention and control of iodine deficiency in pregnancy, lactation and in children less than 2 years of age held in 2005 set the adequate MUIE of > 150 µg/L during pregnancy and lactation, made recommendations to assess and categorize the level of implementation of salt iodization program and, based of this analysis, complementary strategies should be considered [8]. Nowadays there is a growing trend from learned societies that supplementation in pregnancy should be used in countries with mild-to-moderate iodine deficiency, although some scientific organizations recommend that all pregnant and breastfeeding women should take at least 150 µg iodine supplementation, not only in iodine deficient regions but also in iodine sufficient areas. But there are not adequate randomized control trials (RCTs) on the impact of mild-to-moderate iodine insufficiency on pregnancy outcomes and child neurodevelopment and the benefits of correcting this condition in pregnant women is a debate of matter.
The aim of this study was to obtain longitudinal data on urinary iodine excretion and the changes of maternal thyroid parameters (thyroid volume and serum total T4 and TSH) in two groups of healthy women with MUIE < 150 µg /L and those with MUIE ≥ 150 µg/L residing in an iodine replete area of Tehran. The results of this study will add further knowledge regarding the impact of iodine nutrition by universal salt iodization (USI) on feto-maternal thyroid parameters in pregnant women residing in sufficient iodine intake of general population for more than one decade.
Subjects
The present study is part of a cohort study, investigating the relative influences of iodine intake on thyroid size and function of mothers and their infants during and after pregnancy.
A sample size calculation was used and a total of 500 pregnant women enrolled from two mother-child health care centers in Tehran, capital city of IR. Iran. Thirty five individuals discontinued study and finally 465 individuals enrolled in the study and the same women were followed during three trimesters of pregnancy. At initial presentation, during the first trimester, after obtaining written informed consent from each subject, three separate urine samples were obtained during one week for measurement of urinary iodine excretion and based on the MUIEs of these three urine samples, the cohort was divided in two groups: Group I: subjects with MUIE < 150µg/L, and group II: subjects with MUIE ≥ 150 µg/L.
Ultrasonic thyroid volume measurement, urinary iodine excretion and thyroid function tests were measured sequentially in all women during the three trimesters (T) of pregnancy (T1, n = 465, T2, n = 400, T3, n = 306). All pregnant women were singleton-pregnant and delivered live singleton normal infants and received no iodine-containing supplements during pregnancy.
Methods
Thyroid volume was measured according to Brunn, et al. [9] using Aloka SSD-500 (Tokyo-Japan) portable ultrasound unit with a standard 5.0 MHz transducer; for each lobe, the maximum antero-posterior dimension (width) and medio-lateral dimension (thickness) were measured on a transverse image and the maximum length was measured on a longitudinal image. The volume of each lobe was calculated by the formula:
Thyroid volume (ml) = width × length × thickness (mm) × 0.48.
Urinary iodine excretion was measured in random urine samples using a manual method based on Sandell-Kolthoff technique [10]. Results were expressed as microgram of iodine per liter of urine (µg/L).
Total and free T4 and T3 were measured by radioimmunoassay (RIA) method and TSH measurement was done by immunoenzymometric assay (IRMA) using commercial kits (Isotop, Budapest, Hungray) with gamma counter (Wallac Wizard, Wallac Oy, Turku, Finland). Intra and inter-assay coefficient of variations (CV) were 3.3% and 6.2% for T4, 6.7% and 7.8% for T3, 3.8% and 5.8% for free T4 and 3.9% and 7.1% for TSH, respectively.
T3-uptake was measured by enzyme immunoassay (EIA) using Pishtazteb kit (Tehran, Iran). The intra and inter-assay CVs for its measurement were 2.5% and 3.3% respectively.
Anti-thyroglobulin (anti-Tg) and anti-thyroid peroxidase antibodies (anti-TPO) were measured by enzymoimmuometric assay (EIMA) using their kits (Orgentec Diagnostica GmbH, Mainz, Germany). Intra and inter- assay CV were 2.2% and 4.9% for anti-Tg and 3.0 and 5.1% for anti-TPO, respectively. All EIA EIMA tests were read by ELISA reader (Sunrise, Tecan Co. Salzburg, Austria).
Statistics
The normality of urinary iodine excretion (UIE), serum total T4, TSH and thyroid volume (TV) in three trimesters of pregnancy was checked using histogram and kolmogrov-Smirnov test. For non-normal distribution variables we employed non-parametric methods for the analysis and data were expressed as median and inter quartile range (IQR), for normal distribution variables data were expressed as mean ± SD. The trend of UIE, serum total T4, TSH and TV in three trimesters was analyzed using Friedman test in each group. To test the difference between two groups in each trimester, we applied Mann-Withney test, using Bonferroni correction for these analysis and p-value less than 0.016 considered as significant, for the rest of analysis, p < 0.05 was considered significant. SPSS Version 20 was used for all data analysis.
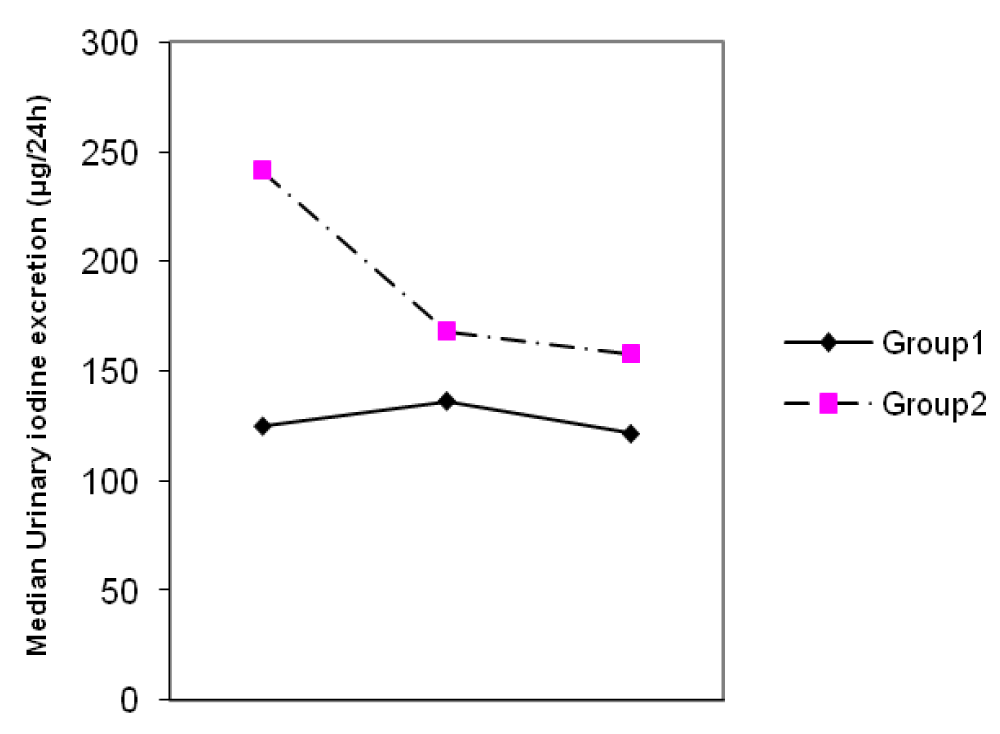
The mean ± SD age of participants was 25.1 ± 5.1 years. The MUIEs in groups I and II in the first, second and third trimesters were 123 and 250 µg/L, 127 and 166 µg/L, 120 and 150 µg/L, respectively. The MUIE in the third trimester of pregnancy in group I did not differ significantly from the values in the first and second trimester (p = 0.67), but it did decline significantly (p < 0.001) in group II (Figure 1).
Figure 1: Changes of median urinary iodine excretion (UIE) during three trimesters of pregnancy.
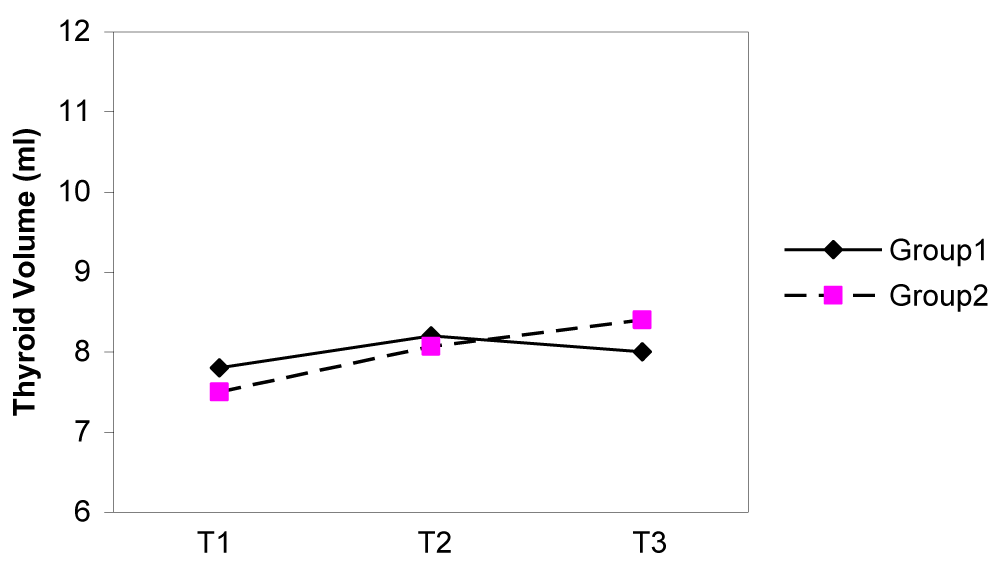
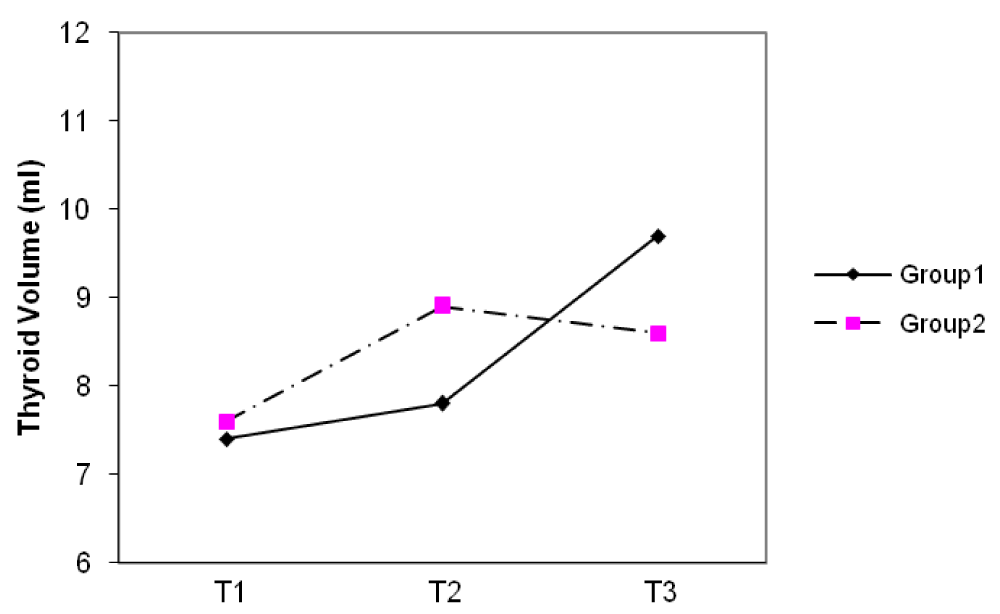
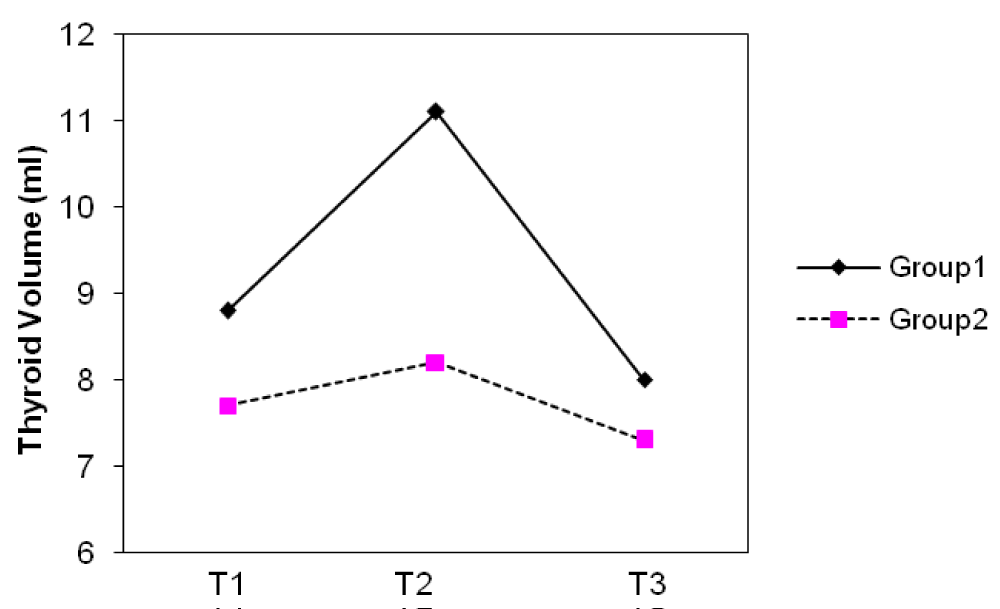
The median thyroid volumes of subjects, in the first, second and third trimesters were 7.8, 8.2 and 8.1 ml in group I and 7.5, 8.0 and 8.4 ml in group II, respectively. In both groups, thyroid volume significantly did increase during pregnancy (p < 0.01) (Figure 2). The median thyroid volumes of subjects with TSH > 2.5 mIU/L in the first, second and third trimester were 8.8 and 7.7 ml , 11.1 and 8.2 ml , 8.0 and 7.3 ml in group I and II, respectively. However, no difference in thyroid volume was found between two groups in each of the three trimesters of pregnancy (Figures 3,4). The mean (± SD) TSH concentration of subjects in the first, second and third trimester was 2.3(± 2.6), 2.1(± 1.8), 2.3(± 1.7) mIU/L in group I and 2.1(± 3.1), 2.1(± 1.8) and 2.0(± 1.3) mIU/L in group II, respectively. In group I, 68.5% had TSH < 2.5 and 31.5% had TSH > 2.5 mIU/L and in group II, 74.8% had TSH < 2.5 and 25.2% had TSH > 2.5 mIU/L in the first trimester of pregnancy. 26.3 % of subjects in group I vs. 13.3% of subjects in group II had changes indicating a rising TSH trends in favor of hyperthyrotropinemia up to the end of pregnancy (p = 0.06, OR = 2.33 , 95% CI = 0.92 – 5.79 ).
Figure 2: Changes of median thyroid volume (TV) during three trimesters of pregnancy.
Figure 3: Changes of median thyroid volume (TV) during three trimesters (T) of pregnancy in subjects with TSH < 2.5 mIU/L.
Figure 4: Changes of median thyroid volume (TV) during three trimesters (T) of pregnancy in subjects with TSH ≥ 2.5 mIU/L.
The mean (± SD) total T4 concentrations of subjects in the first, second and third trimesters were 13.2(± 3.4), 13.8(± 3.3), 13.0(± 2.9) µg/dl in group I and 13.1(± 3.2), 13.7(± 2.9), 13.4 (± 3.2) µg/dl in group II, respectively. There was no correlation between the thyroid volume and three observed parameters (UIE, total T4 and TSH) during the pregnancy in the two groups. Twelve percent in group I and 7% in group II were anti-TPO positive. Median (IQR) of Tg was 10.08(5.7-20.4) and 8.3(3.9-18.2) µg/dl in group I and group II, respectively.
The aim of this prospective study was to obtain information on urinary iodine excretion, and to evaluate changes of maternal thyroid parameters in relation with iodine status in healthy pregnant women with median urinary iodine < 150 µg/L and ≥ 150 µg/L, living in an area with iodine sufficiency in Tehran.
The iodine requirement increases during pregnancy and recommended intake is 250 µg/day [8]. Pregnant women is a group recommended for active assessment of iodine status [11], but monitoring iodine status during pregnancy is a challenge. The recommendation from WHO suggest that a median urinary iodine concentration > 150 µg/L indicates adequate iodine intake in pregnancy [12].
Salt iodization in IR Iran has begun since 1990. The production, distribution and consumption of iodized salt had increased gradually, and the results of national surveys from 1996 to 2014 have shown that more than 95% of Iranian population in urban and more than 92% in rural areas were consuming iodized salt. IR. Iran has fulfilled all 10 programmatic indicators set by WHO/UNICEF/ICCIDD [13] and has achieved a sustainable IDD control program since 1996. This has been recognized by WHO/EMRO in the year 2000 [14]. It has been shown that moderate to severe iodine deficiency adversely affects maternal thyroid parameters and cognition and growth in their children [15-19]. Mild to moderate growth retardation and alteration in auditory, neurologic and psychomotor development have been shown in apparently normal subjects residing in areas of iodine deficiency before iodine supplementation in Iran [20,21]. It had been estimated that populations with chronic iodine deficiency experience a reduction up to 13.5 points in intelligent quotient [22]. Our study in villages with sever iodine deficiency has shown 8 point increase in intelligence quotient 10 years following iodine supplementation [23]. But the potential adverse effects of mild to moderate iodine deficiency during pregnancy are uncertain [24]. The reports of controlled trials of iodine supplementation in mildly iodine deficient pregnant women suggest either no change or a beneficial effects on maternal and newborn thyroid parameters [25-29]. MUIE of subjects in these trials was between 30 to 75 µg/L at the beginning of the studies but supplementation of iodine (between 50 to 230 µg/day) significantly increased maternal UIE (between 100-130 µg/L) in all trials. The effect of iodine supplementation on maternal and newborn thyroid parameters in these trials was challengeable. In Romano, et al. study [25], the maternal median urinary iodine increased from 34 µg/L to 100µg/L by giving 120-180 µg iodine/day but thyroid volume did not change and treatment had no effect on maternal TSH.
In Pedersen, et al. study [26], the maternal UIE increased from 55 µg/L to 90-110 µg/L by supplementation of 200 µg iodine/day. This group had less thyroid volume increment and lower serum Tg and TSH than control group, but no significant differences were found between groups for T3 and T4. Glinor, et al. [27] supplemented pregnant women with 100 µg iodine/day. In this study, by increasing UIE in treated group, thyroid volumes and TSH showed significant differences but there was not significant difference for T3, FT4 and T3/T4 ratio. In Liesenkotter, et al. study [28], treatment with 230 µg iodine/day and increment of MUIE to 104 µg/L had significant effect on maternal TSH, T3, T4 and thyroid volume. In Antonangeli, et al. study [29] there were no differences in maternal FT4, FT3, TSH and thyroid volume between treated groups with 200 µg iodine/day (median UIE 230 µg/L) and with 50 µg iodine/day (median UIE 128 µg/L). Zimmerman, et al. [30] also have demonstrated that supplementation had no effect on maternal and newborn total or free thyroid hormone concentrations.
It has recently been proposed to use “trimester-specific” reference ranges for serum TSH levels during pregnancy and serum TSH levels above 2.5 mIU/L may be indicative of a slight thyroid under-function [31-33]. In our study, the mean serum TSH did not change significantly during three trimesters of pregnancy in those with median UIE ≥ 150 µg/L and those with median UIE < 150 µg/L, which is compatible with some investigation in this regards (25,26,29,30). 26.7% of subjects in group I vs. 13.3% in group II had changes indicating a rising TSH trends in favor of hyperthyrotropinemia up to the end of pregnancy, although this difference was not statistically significant (p = 0.06), but regarding to the relative risk (OR= 2.33, 0.95% CI = 0.92- 5.79 ), it seems that in women with UIE < 150 / µg L, living in iodine sufficient areas , the occurrence of hyperthyrotropinemia is more likely which could propel them and their infants toward the thyroid dysfunction.
The median thyroid volume showed significant differences during three trimesters of pregnancy in either groups. UIE also showed significant difference only in iodine sufficient group. There are a few known factors influencing the thyroid volume. Iodine supply, TSH concentration, anthropometric parameters, sex, age, parity and genetic factors are the most important determinants of the thyroid gland size [34,35]. Thyroid volume and urinary iodine excretion are the function of the ambient iodine intake. Renal loss of iodine during pregnancy, has been suggested as the cause of goiter in pregnant women [36], although this is unclear [37,38]. It has been shown that thyroid volume increases during pregnancy in iodine deficient areas but there was no difference in sonographic measured thyroid volume between pregnant and non-pregnant women in iodine replete areas [39-42].
In regions with a sufficient iodine intake, changes in thyroid volume remained minimal (10% - 15% on the average), but in regions with a lower iodine intake, the changes were much larger, with thyroid volume increments ranging between 20% - 30% on the average [43-46]. Change of thyroid volume in iodine sufficient areas, has been attributed mainly to vascular thyroid swelling during pregnancy [47,48].
In our study, mild increments of thyroid volume in either groups might be due to vascular thyroid swelling but the change of TSH between two groups is considerable because in 26.7% of women with UIE < 150 µg L, TSH did rise to more than 2.5 mIU/L up to the end of pregnancy and this value for women with UIE ≥ 150 µg /L was 13.3%, although this difference is not statistically significant but it is challengeable.
In summary, this study is the only investigation in which two groups of pregnant women were selected according to the recommendation of WHO/ICCIDD/UNICEF based on their UIE from an area with iodine sufficiency where more than 95% of household are consuming iodized salt and the results indicate that even in areas with well-established universal salt iodization program, pregnancy could be a risk of having iodine deficiency and despite WHO/ICCIDD/UNICEF recommendation which believe that dietary iodine fortification during pregnancy depends primarily on the extent of pre-existing iodine deprivation [49], systematic dietary fortification needs to be implemented in this vulnerable group.
This research project has been supported by grants from Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences.
- Delange F. The role of iodine in brain development. Proc Nutr Soc. 2000; 59: 75-79. PubMed: https://pubmed.ncbi.nlm.nih.gov/10828176/
- Glinoer D. Iodine nutrition requirements during pregnancy. Thyroid. 2006; 16: 947-948. PubMed: https://pubmed.ncbi.nlm.nih.gov/17042676/
- Dafnis E, Sabatini S. The effect of pregnancy on renal function: physiology and pathophysiology. Am J Med Sci. 1992; 303: 184-205. PubMed: https://pubmed.ncbi.nlm.nih.gov/1595782/
- Smyth PP. Variation in iodine handling during normal pregnancy. Thyroid. 1999; 7: 637-642. http://www.ncbi.nlm.nih.gov/pubmed/10447006
- Berghot A, Wiersinga WM. Thyroid size and thyroid function during pregnancy. In: Stanbury JB, Delange F, Dunn JT,Pandav CS, eds. Iodine in pregnancy. Delhi. Oxford University Press. 1998: 35-53.
- Glinor D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocrine Rev. 1977; 18: 404-433. PubMed: https://pubmed.ncbi.nlm.nih.gov/9183570/
- Hetzel B, Pandav C. S.O.S for a Billion: The Conquest of Iodine Deficiency Disorders. Oxford University Press. New York, NY. 1994.
- Reaching optimal iodine nutrition in pregnant and lactating women and young children. IDD newsletter. 2008; 27.
- Brunn J, Block U, Ruf G, Bos I, Kunze WP, et al. Volumetric der schildrusenlappen mittels real-time-sonographie. Deutsche Medizinische Wochenschrift. 1981; 106: 1338-1340.
- Sandell EB, Kolthoff IM. Micro determination of iodine by a catalytic method. Mikrochemica Acta. 1937; 19-25.
- Stanbury JB, Delange F, Dunn JT, Pandav CS. Iodine in pregnancy. New Dehli: Oxford University Press. 1998; 1-297.
- Proceedings of the WHO Technical Consultation on control of iodine deficiency in pregnant women and young children. Geneva. 2005.
- Azizi F. Assessment, monitoring and evaluation of iodine deficiency disorders in the middle east and eastern mediterranian region. Tehran, Sara publication. 2002.
- WHO, Promotion of iodized salt in the Eastern Mediterranean Region, Middle East and North Africa: Report of an inter country meeting, Dubai, united Arab Emirates. 2000.
- Delong GR, Robbins J, Condliffe PG. Iodine and the brain. New York: Plenum Press. 1989; 1-379.
- Stanbury JB. The damaged brain of iodine deficiency. New York: Cognizant Communication Co. 1994: 1-335.
- Morreale de Escobar G, Obregón MJ, Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab. 2000; 85: 3975-3987. PubMed: https://pubmed.ncbi.nlm.nih.gov/11095417/
- Delange F. Iodine deficiency as a cause of brain damage. Postgrad Med J. 2001; 77: 217-220. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1741987/
- Lavado-Autric R, Ausó E, García-Velasco JV, Arufe Mdel C, Escobar del Rey F, et al. Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest. 2003; 111: 1073-1082. PubMed: https://pubmed.ncbi.nlm.nih.gov/12671057/
- Azizi F, Sarshar A, Nafarabadi M, Ghazi A, Kimiagar M, et al. Impairment of neuromotor and cognitive development in iodine-deficient schoolchildren with normal physical growth. Acta Endocrinol (Copenh). 1993; 129: 501-504. PubMed: https://pubmed.ncbi.nlm.nih.gov/8109182/
- Azizi F, Kalani H, Kimiagar M, Ghazi A, Sarshar A, et al. Physical, Neuromotor and intellectual impairment in non-cretinous schoolchildren with iodine deficiency. Int J Vit Nutr Res. 1995; 65: 199-205. PubMed: https://pubmed.ncbi.nlm.nih.gov/8830000/
- Bleichrodt N, Born MP. A metaanalysis of research on iodine and its relationship to cognitive development. In: Stanbury JB(ed) The Damaged Brain of Iodine Ddficiency. Cognizant Communication, New York. 1994; 195-200.
- Salarkia N, Hedayati M, Mirmiran P, Kimiagar M, Azizi F. Evaluation of the impact of an iodine supplementation program on severely iodine-deficient schoolchildren with hypothyroidism. Public Health Nutr. 2003; 6: 529-533. PubMed: https://pubmed.ncbi.nlm.nih.gov/14690034/
- Bleichrodt N, Escobar del Rey F, Morreale de Escobar G, et al. Iodine deficiency Implications for mental and psychomotor development in children. In: Iodine and the Brain (Editors: DeLong GR, Robbins G, Condliffe PG). Plenum Press. New York. 1989; 269.
- Romano R, Jannini EA, Pepe M, Grimaldi A, Olivieri M, et al. The effects of iodoprophylaxis on thyroid size during pregnancy. Am J Obstet Gynecol. 1991; 164: 482-485. PubMed: https://pubmed.ncbi.nlm.nih.gov/1992688/
- Pedersen KM, Laurberg P, Iversen E, Knudsen PR, Gregersen HE, et al. Amelioration of some pregnancy-associated variations in thyroid function by iodine supplementation. J Clin Endocrinol Metab. 1993; 77: 1078-1083. PubMed: https://pubmed.ncbi.nlm.nih.gov/8408456/
- Glinoer D, De Nayer P, Delange F, Lemone M, Toppet V, et al. A randomized trial for the treatment of mild iodine deficiency during pregnancy: maternal and neonatal effects. J Clin Endocrinol Metab. 1995; 80: 258-269. PubMed: https://pubmed.ncbi.nlm.nih.gov/7829623/
- Liesenkötter KP, Göpel W, Bogner U, Stach B, Grüters A. Earliest prevention of endemic goiter by iodine supplementation during pregnancy.Eur J Endocrinol. 1996; 134: 443-448. PubMed: https://pubmed.ncbi.nlm.nih.gov/8640295/
- Antonangeli L, Maccherini D, Cavaliere R, Di Giulio C, Reinhardt B, et al. Comparison of two different doses of iodide in the prevention of gestational goiter in marginal iodine deficiency: a longitudinal study. Eur J Endocrinol. 2002; 147: 29-34. PubMed: https://pubmed.ncbi.nlm.nih.gov/12088916/
- Zimmermann M, Delange F. Iodine supplementation of pregnant women in Europe: a review and recommendations. Eur J Clin Nutr. 2004; 58: 979-984. PubMed: https://pubmed.ncbi.nlm.nih.gov/15220938/
- Panesar NS, Li CY, Rogers MS. Reference intervals of thyroid hormones in pregnant Chinese women. Ann Clin Biochem. 2001; 38: 329-332. PubMed: https://pubmed.ncbi.nlm.nih.gov/11471873/
- Haddow JE, Knight GJ, Palomaki GE, McClain MR, Pulkkinen AJ. The reference range and within-person variability of thyroid stimulating hormone during the first and second trimesters of pregnancy. J Med Screen. 2004; 11: 170-174. PubMed: https://pubmed.ncbi.nlm.nih.gov/15563772/
- Dashe JS, Casey BM, Wells CE, McIntire DD, Byrd EW, et al. Thyroid stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet Gynecol. 2005; 106: 753-757. PubMed: https://pubmed.ncbi.nlm.nih.gov/16199632/
- Hansen PS, Brix TH, Bennedbaek FN, Bonnema SJ, Kyvik KO, et al. Genetic and environmental causes of individual differences in thyroid size: a study of healthy Danish twins. J Clin Endocrinol Metab. 2004; 89: 2071-2077. PubMed: https://pubmed.ncbi.nlm.nih.gov/15126523/
- Berghout A, Wiersinga WM, Smits NJ, Touber JL. Determinants of thyroid volume as measured by ultrasonography in healthy adults in a non-iodine deficient area.Clin Endocrinol (Oxf). 1987; 26: 273-280. PubMed: https://pubmed.ncbi.nlm.nih.gov/3308184/
- Glinoer D. Pregnancy and iodine. Thyroid. 2001; 11: 471-481. PubMed: https://pubmed.ncbi.nlm.nih.gov/11396705/
- Nelson M, Wickus GG, Caplan RH, Beguin EA. Thyroid gland size in pregnancy. An ultrasound and clinical study. J Reprod Med. 1987; 32: 888-890. PubMed: https://pubmed.ncbi.nlm.nih.gov/3323500/
- Hegedüs L. Thyroid size determined by ultrasound. Influence of physiological factors and non-thyroidal disease. Dan Med Bull. 1990; 37: 249-263. PubMed: https://pubmed.ncbi.nlm.nih.gov/2192837/
- Crooks J, Tulloch MI, Turnbull AC, Davidsson D, Skulason T, et al. Comparative incidence of goiter in pregnancy in Iceland and Scotland. Lancet. 1967; 2: 625-627.
- Levy RP, Newman DM, Rejali LS, Barford DA. The myth of goiter in pregnancy. Am J Obstet Gynecol. 1980; 137: 701-703. PubMed: https://pubmed.ncbi.nlm.nih.gov/7395933/
- Long TJ, Felice ME, Hollingsworth DR. Goiter in pregnant teenagers. Am J Obstet Gynecol. 1985; 152: 670-674. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3875289
- Liberman C, Pino SC, Fang SL, Braberman LE, Emerson CH. Circulating iodide concentrations during and after pregnancy. J Clin Endocrinol Metab. 1998; 83: 3545-3549. PubMed: https://pubmed.ncbi.nlm.nih.gov/9768662/
- Berghout A, Wiersinga W. Thyroid size and thyroid function during pregnancy: an analysis. Eur J Endocrinol. 1998; 138: 536-542. PubMed: https://pubmed.ncbi.nlm.nih.gov/9625365/
- Rasmussen NG, Hornnes PJ, Hegedüs L. Ultrasonographically determined thyroid size in pregnancy and post partum: the goitrogenic effect of pregnancy. Am J Obstet Gynecol. 1989; 160: 1216-1220. PubMed: https://pubmed.ncbi.nlm.nih.gov/2658612/
- Smyth PP, Hetherton AM, Smith DF, Radcliff M, O'Herlihy C. Maternal iodine status and thyroid volume during pregnancy: correlation with neonatal iodine intake. J Clin Endocrinol Metab. 1997; 82: 2840-2843. PubMed: https://pubmed.ncbi.nlm.nih.gov/9284707/
- Romano R, Jannini EA, Pepe M, Grimaldi A, Olivieri M, et al. The effects of iodoprophylaxis on thyroid size during pregnancy. AM J Obstet Gynecol. 1991; 164: 482-485. PubMed: https://pubmed.ncbi.nlm.nih.gov/1992688/
- Fister P, Gaberscek S, Zaletel K, Krhin B, Gersak K, et al. Thyroid volume and intrathyroidal blood flow increase during pregnancy. Clin Endocrinol (Oxf). 2006; 65: 828-829. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17121539
- Bogazzi F, Bartalena L, Brogioni S, Burelli A, Manetti L, et al. Thyroid vascularity and blood flow are not dependent on serum thyroid hormone levels: studies in vivo by color flow doppler sonography. Eur J Endocrinol. 1999; 140: 452-456. PubMed: https://pubmed.ncbi.nlm.nih.gov/10229913/
- Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years old: conclusions and recommendations of the Technical consultation. Public Health Nutrition. 2007; 10: 1606-1611. PubMed: https://pubmed.ncbi.nlm.nih.gov/18053287/