More Information
Submitted: November 04, 2022 | Approved: November 15, 2022 | Published: November 16, 2022
How to cite this article: Lahari N, Shrivastava VK, Sreenikethanam A, Raj S, Bajhaiya AK. Interdictory contribution of Vitamin D to prevent corona virus infections. Arch Food Nutr Sci. 2022; 6: 073-081.
DOI: 10.29328/journal.afns.1001041
Copyright License: © 2022 Lahari N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Vitamin D; COVID-19; Respiratory infection; Immune system; Hypovitaminosis D; Vitamin D receptor
Interdictory contribution of Vitamin D to prevent corona virus infections
Neelam Lahari1*, Vinoy K Shrivastava1, Arathi Sreenikethanam2, Subhisha Raj2 and Amit K Bajhaiya2*
1Laboratory of Endocrinology, Department of Bioscience, Barkatullah University, Bhopal, Madhya Pradesh, India
2Algal Biotechnology Lab, Department of Microbiology, School of Life Sciences, Central University of Tamil Nadu, Thirvarur, India
*Address for Correspondence: Amit K Bajhaiya, Algal Biotechnology Lab, Department of Microbiology, School of Life Sciences, Central University of Tamil Nadu, Thirvarur, India, Email: [email protected]
Neelam Lahari, Laboratory of Endocrinology, Department of Bioscience, Barkatullah University, Bhopal, Madhya Pradesh, India, Email: [email protected]
The impact of vitamin D on the musculoskeletal system is well known. The diverse role of vitamin D is well supported by the functionality of vitamin D receptors and vitamin D activating enzymes (hydroxylase) present in tissues and cells. Hypovitaminosis D causes rickets, osteomalacia, hyperparathyroidism, and an increased risk of bone fracture. Vitamin D has immune-stimulatory effects on both the innate and adaptive immune systems. Vitamin D induces antimicrobial peptide cathelicidin and defensin that can inhibit viral replication of pro-inflammatory cytokines that regulate inflammatory encasement. Moreover, several studies on vitamin D have shown its interdictory role in the immune and respiratory systems. This global crisis, the COVID-19 pandemic condition has increased the risk of acute respiratory tract infection by immune dysregulation along with cytokine storm, which further progress into acute respiratory distress syndrome. Vitamin D has immunomodulatory and anti-inflammatory properties which are effective against respiratory viral infections. Vitamin D supplementation has shown a compatible effect on viral infection. This review article discusses the role of vitamin D in reducing the risk of respiratory infections including the severity of COVID-19 infections. This review focuses on the therapeutic role of vitamin D to improve clinical outcome during COVID-19 infection and suggest its possible role in the prevention and treatment of respiratory infections.
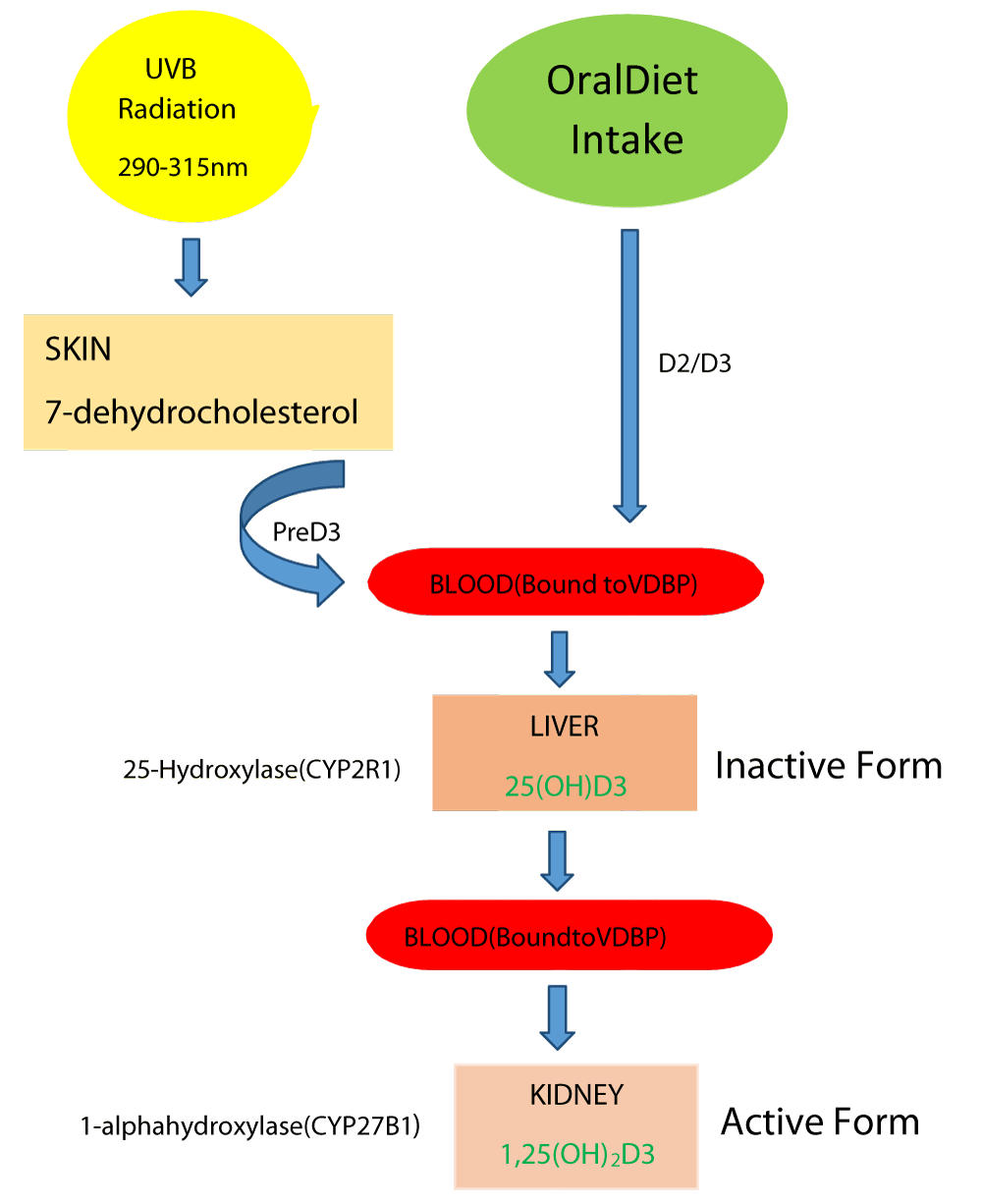
Vitamin D is a secosteroid that can either be produced in the skin from a cholesterol-like precursor 7-dehydrocholesterol when exposed to sunlight (ultraviolet B radiation 290 nm - 315 nm) or by providing the precursors in the diet. The version made in the skin is referred to as vitamin D3 (cholecalciferol), whereas the dietary form can be vitamin D3 or a closely related molecule of plant origin known as vitamin D2 (ergocalciferol) [1,2]. As vitamin D can be made in the skin, some nutritional texts refer to it as a pro-hormone. It’s carried in the bloodstream by vitamin D binding protein (VDBP) and is hydroxylated at C-25 by cytochrome P450. Further the 25-hydroxylase (CYP2R1) enzyme present in the liver results in the formation of the main circulating inactive form 25-hydroxyvitamin D [25(OH)D] (Calcidiol). The 1,25-hydroxylase (CYP27B1) enzyme converts this metabolite to the biologically active form 1,25-dihydroxy vitamin D [1,25(OH)2D] (Calcitriol) in the kidney [3,4] (Figure 1). Several factors affect the vitamin D levels such as age, being indoors, dark skin, pollution, sunscreen uses, and low cholesterol levels. All these factors negatively regulate vitamin D biosynthesis in the skin [5]. The 25(OH)D is used as an indicator to determine the vitamin D levels in the body as it depends on the availability and circulating vitamin D in the blood. According to the classification given by the US Endocrine Society, < 20 ng/ml of serum 25(OH)D with a consequent and consistent elevation of parathyroid hormone and decrease in intestinal calcium absorption is considered to be vitamin D deficiency, > 30 ng/ml is sufficient and 21 ng/ml - 29 ng/ml is considered as an insufficient condition. The World Health Organization (WHO) also defined vitamin D insufficiency as a serum level of 25(OH)D below 20 ng/ml [6-8] (Tables 1,2).
Figure 1: Diagrammatic representation of Vitamin D synthesis: Exposure to sunlight relay photolysis of the 7-dehydrocholesterol in the skin, which is converted to previtamin D3 by a heat-dependent process. Vitamin D made in the skin or ingested in the diet is converted to 25(OH)D3 (inactive form) by CYP2R1 in the liver, then 25(OH)D3 converted 1,25(OH)2D3 (active form) by CYP27B1 in the kidney [1,4].
| Table 1: Blood serum status of vitamin D [25(OH)D] (Adapted from [70]). | ||
| Vitamin D Status | 25(OH)D–ng/ml | 25(OH)D-nmol/L |
| Sufficiency | > 30 | 75 |
| Insufficiency | 21 - 29 | 51 - 74 |
| Deficiency | < 20 | < 50 |
| Table 2: Classification of Vitamin D deficiency (Adapted from [7]). | ||
| Stage | Vitamin D status(ng/ml) | Vitamin D status(nmol/L) |
| Severe Deficiency | <5 | < 12.5 |
| Moderate Deficiency | 5-10 | 12.5 - 25 |
| Mild Deficiency | 10-20 | 25 - 50 |
At the biochemical level, 1,25(OH)2D binds to the nuclear receptor superfamily member called the vitamin D receptor (VDR). VDR mediates the biological actions of vitamin D to maintain mineral homeostasis in the intestine, kidney, bone, and parathyroid gland. But its deficiency can lead to certain disorders like rickets in children, osteoporosis in adults, muscle weakness, certain cancers, multiple sclerosis, diabetes, blood pressure, and another physiological process [9,10]. Further vitamin D is also known to play role in the immune system as VDR is expressed in immune cells (monocytes/macrophages, dendritic cells, T cells, B cells, NK cells, etc.) as well [11]. The expression of the VDR receptor in immune cells has highlighted an interesting role of vitamin D in the immune response. Recent data demonstrate a link between vitamin D and various infectious diseases caused by different pathogens. Further, many studies have suggested that vitamin D can reduce the risk of viral diseases [12-14].
The current Corona Virus Disease- 19 (COVID-19) was an epidemic and was detected first in Wuhan city, China in December 2019. The 2019-CoV was later named SARS-CoV2 (Severe Acute Respiratory Syndrome Corona Virus 2). On11th of February 2020, WHO named it COVID-19.
As the number of COVID cases increased rapidly worldwide [15-19]. So, WHO declared it a global pandemic on the 11th of March 2020 [http//www.who.int]. The main symptoms of COVID-19 were fever, dyspnea (shortness of breath), cough, sore throat, nasal congestion, bone & muscle aches, headache, and fatigue [20-25]. The corona symptoms were very much similar to that of viral pneumonia and common influenza infections. Human to Human transmission is implicated with droplets as the main mode of transmission. In some cases like asymptomatic or with mild symptoms, the infection is referred to as Acute Respiratory Distress Syndrome (ARDS). In severe cases like a respiratory failure along with multi-organ dysfunction, COVID infected person shows a preferment of cytokines such as interleukin (IL) -6, 10 and tumor necrosis factor (TNF)-α [26,27]. So, the viral infection provokes tissue injury through increased production of pro-inflammatory cytokines, mobilization of pro-inflammatory macrophages, granulocytes, and activation of T cells. This phenomenon was commonly known as cytokine storm and is also referred to as macrophage activation syndrome. This cytokine storm was considered the main reason for some of the serious manifestations of COVID-19. Some of the recent studies on COVID-19 showed that the infection was mainly associated with increased production of pro-inflammatory cytokines, C-reactive protein, causing an increased risk of pneumonia, sepsis, ARDS, and heart failure [28-30]. Vitamin D also plays an immuno-modulatory role via suppression of immune responses in respiratory epithelial cells during viral infections. Vitamin D reduces the levels of pro-inflammatory cytokines including IL-1, IL-6, IL-12, TNF-α and IL-17, while increasing the anti-inflammatory IL-10 and TNF-β. Several studies showed that vitamin D deficiency can detract from the zone of acquired immunity. In this review, we discuss the innovative source and defensive role of vitamin D against coronavirus infections [30-33].
Immuno-regulatory action of cholecalciferol
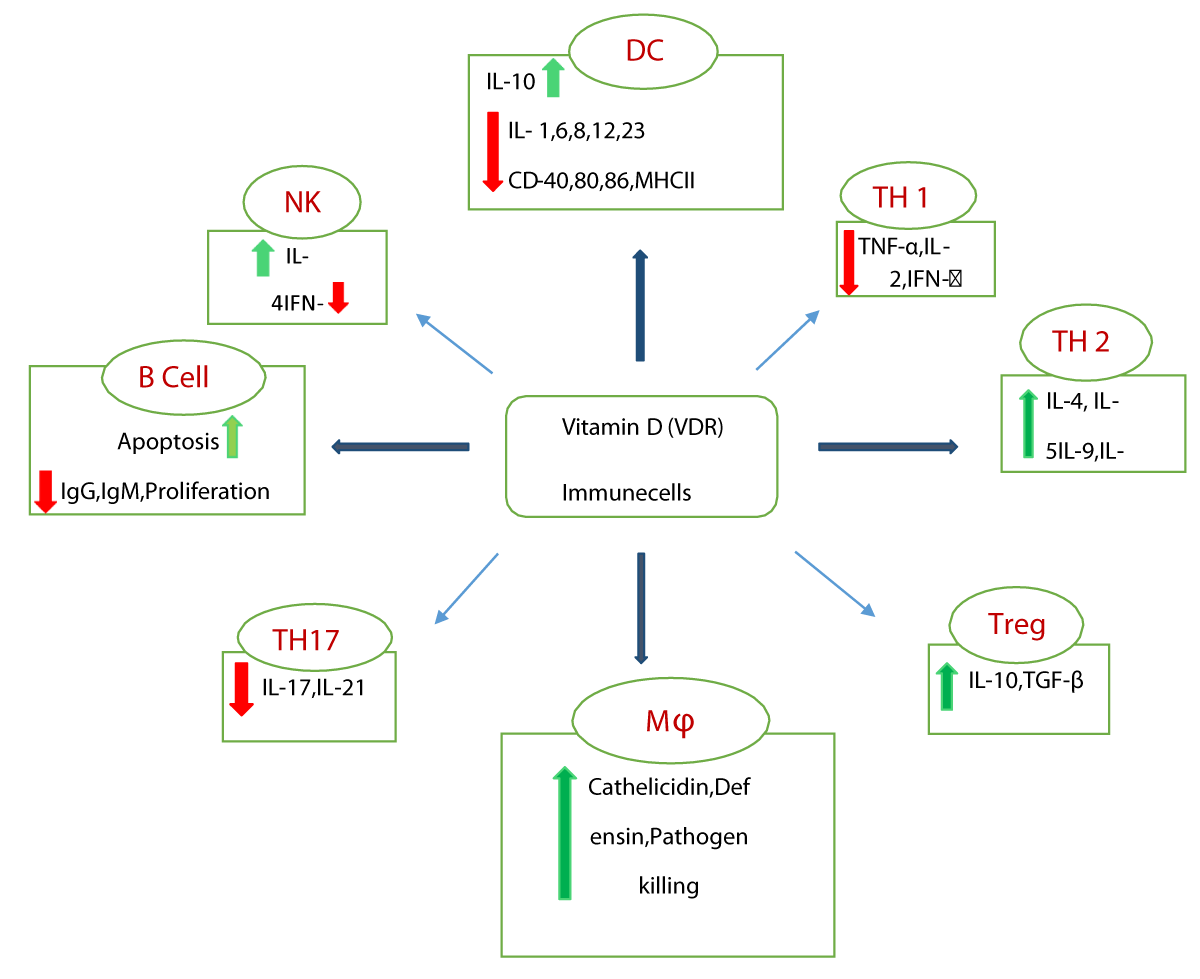
The contribution of vitamin D as an immune-modulator or immune-regulator has been the subject of interest among immunologists. The importance of vitamin D in the regulation of the immune system (innate and adaptive) was demonstrated by the discovery of the presence of metabolizing hormones and VDR expression in almost all immune cells [34,35]. The effect of vitamin D on immune cells is complex as illustrated by the fact that VDR expression in immune cells is differently controlled according to their activation status. Inside the cell, vitamin D binds to the nuclear D receptor and subsequently activates VDRs to dimerize with themselves or with retinoid X receptor (RXR) and translocate to the nucleus to engage the vitamin D receptor element (VDRE) [36-40]. VDRE regulates the expression of numerous host genes like β defensin and cathelicidin. These can directly cleave the membrane of a virus and are involved in the activation of different immune cells. Vitamin D suppresses responses of adaptive immunity in respiratory epithelial cells during viral infections [41-43]. It reduces the Th 1 proliferation that results in lower levels of pro-inflammatory cytokines and diminished acquired immune responses and these may be counter-protective in mounting a successful immune response against a virus [44,45] (Figure 2).
Figure 2: Schematic representation of the defense mechanism of Vitamin D in Immune Cells. DC: Dendritic Cells; TH: T Helper Cells; Mϕ: Macrophage; Treg: T regulator cell; NK: Natural Killer Cells; red arrow indicate-downregulation, green arrow indicate-upregulation.
Vitamin D influences T-cell maturation and can divert the development of inflammatory T-helper cells. 1,25(OH)2D and suppresses the responses mediated by Th 1 by repressing the production of inflammatory cytokines IL-2, IFN-γ, and TNF-α. Additionally, vitamin D promotes cytokine production by Th-2 cells (IL-4, IL-5). Vitamin D also promotes the induction of the T regulatory cells, which help to inhibit inflammatory processes and these cells promote the production of IL-10 and TGF-β [46-50]. Vitamin D affects dendritic cells by down-regulating MHC-II, costimulatory molecules (CD-40, 80,86), IL-12, IL-6, IL-8, IL-1, and upregulating IL-10, CD-74. Another target cell of vitamin D is the macrophage/monocyte, which is responsible for antibacterial and antiviral defense by promoting the secretion of antimicrobial products such as cathelicidin (hCAP18 and LL37) and β defensin. Cathelicidin neutralizes endotoxins and helps to reduce viral infections [51-59]. Furthermore 1,25(OH)2D, VDR complex acts on cathelicidin gene promotor vitamin D response elements to enhance transcription of cathelicidin [60-63]. The antiviral responses produced by host immunity try to limit viral spreading, and inflammation and remove infected cells. Vitamin D involves in immune system functions in response to viral infection generating both pro-inflammatory and anti-inflammatory cytokines. Further, these cytokine productions are down-regulated and upregulated against any foreign pathogen [64-68].
Clinch between hypovitaminosis-D and COVID-19
The recently evaluated mean levels of vitamin D in the population across ~40 countries, show more than 50% vitamin D deficiency [69]. The pandemic COVID-19 affected countries including India, which has a substantial population (~ > 75%) of vitamin D deficient. This deficit can be attributed due to major factors like sunlight avoidance, poor diet, and improper lifestyle [70,71]. In Europe, ~ 45% of the population is deficient in Vitamin D, and prevalence is higher in dark-skinned people compared to white sinned. Similarly, ~ 24% of US citizens and ~ 37% of Canadians are deficient in vitamin D [72-74]. Studies have recognized the association between vitamin D deficiency and viral respiratory infections. Meantime, systematic review and meta-analysis have concluded that vitamin D has potential in respiratory tract infections, especially in those who have a high level of Hypovitaminosis-D [75,76]. There could also be other correlations between vitamin D deficiency and health issues such as diabetes, hypertension, reproduction, and viral infections because vitamin D receptor is distributed in different tissues in the body [77-85]. In some clinical studies, low levels of serum vitamin D were associated with acute respiratory tract infections like influenza and the risk of community-acquired pneumonia. Some retrospective studies determined the correlation of vitamin D status with the severity and mortality of COVID-19 [86-88].
The SARS-CoV2 infects pulmonary epithelial cells using angiotensin-converting enzyme2 (ACE) receptors. Further, the macrophages, neutrophils, and T cells get activated through sustained elevation of cytokines including IL-1, IL-6, and TNF-α resulting in acute respiratory distress syndrome [89-91]. Vitamin D enhances the production of antimicrobial peptides like cathelicidin and β-defensin, which are key factors in immune responses in many respiratory diseases. The cytokines and antimicrobial peptides are responsible for some serious manifestations of COVID-19. But some recent studies have shown vitamin D enhances the expression of ACE2 and increase vascular endothelial growth factor production. A possible role of vitamin D in coronavirus infection based on its impact on innate immunity, adaptive immunity, and rearrangement of the immune response [92-95]. The multicentric study has suggested that whilst COVID-19 patients (vitamin D deficient) generally had poor outcomes, those with high levels of vitamin D fared better outcomes [96,97]. A review has concluded that there was substantial ecological evidence to correlate hypovitaminosis D with the severity of COVID-19 infections [98-100]. A study has observed that African Americans with vitamin D deficiencies as well as those with poorer COVID-19 outcomes may stand to benefit from supplementation. All the above studies suggested that vitamin D supplementation may help patients with COVID-19 [101-105].
The relationship of vitamin D with oxidative stress and cell apoptosis in COVID 1,25(OH)2D is involved in a variety of intracellular genomic activities as well as biochemical and enzymatic reactions, 25(OH)D concentrations are crucial for preventing inflammation, eliminating parasites and microbes that have invaded the body, reducing oxidative stress after routine exposure to toxins, and managing the aging process. For instance, a healthy level of 25(OH)D increases the expression of the nuclear factor erythroid-2(Nf-E2)-related factor 2(Nrf2) as well as Klotho, a hormone that regulates phosphate levels and an antiaging protein. Additionally, it aids in protein stability [106]. The production of antioxidants is one of the cellular signaling systems that Klotho also controls. Therefore, the Klotho gene knockout method or functional defects of the Klotho gene in mice cause premature aging syndrome. Ineffective Klotho and/or FGF23 expression has been demonstrated to accelerate aging in animal experiments. One of the things that make this cycle of oxidative stress stronger and speed up premature cell death is a vitamin D deficit. Numerous intracellular oxidative stress-related activities are downregulated, and vitamin D level is acceptable. Serum 25(OH)D levels that are below ideal levels fail to control oxidative stress, increase intracellular oxidative damage, or slow down the pace of apoptosis. The level of intracellular Nrf2 is inversely linked with the buildup of mitochondrial ROS and the subsequent increase in oxidative stress. Thus, Nrf2 is essential for protecting cells from oxidative stress, which vitamin D regulates. The general positive effects of calcitriol include the upregulation of the expression of several antioxidant and anti-inflammatory cytokines, which shields the tissues from toxins, abnormalities caused by micronutrient deficiencies, and parasite- and intracellular microbe-induced damage. Through its anti-inflammatory properties and the mitochondrial-based expression of antioxidants via the cell signaling pathway, it controls ROS levels. Calcitriol controls the numerous roles of several of the genes in the Klotho-Nrf2 regulatory system. These include boosting the number of intracellular antioxidants, preserving redox equilibrium, and restoring the normal intracellular environment by eliminating excess ROS and so reducing oxidative stress. In addition, the expression of the important redox agent glutathione reductase, glutamate cysteine ligase, and -glutamyl transpeptidase is dependent on vitamin D. Additionally, vitamin D stimulates the production of glutathione peroxidase, which breaks down the ROS molecule H2O2 into water. Through the activation of the enzyme glucose-6-phosphate dehydrogenase, vitamin D also influences the production of glutathione by upregulating superoxide dismutase and downregulating nitrogen oxide, a potent precursor for the production of reactive oxygen species (ROS) that convert oxygen into H2O2 (SOD). Together, these vitamin D-related effects lessen the load of intracellular ROSs [107,108].
Microalgae as a potential source of vitamin D
Vitamin D plays a major role in the human body and is involved in maintaining calcium homeostasis. Moreover, its deficiency is associated with several disorders like osteoporosis, diabetes, cancer, hypertension, and autoimmune diseases [109]. So, it is very important to maintain Vitamin D levels in the body. Vitamin D is naturally synthesized in the skin when exposed to UVB radiations by chemical conversion of the precursors [110]. Limited exposure to sunlight, application of sunscreens, protective clothing, aging, and dark skin are some of the factors which affect the synthesis of Vitamin D in adequate amounts. So, the dietary supplementation of Vitamin D is essential to protect the body from the adverse effects of vitamin D deficiency [111]. Vitamin D is naturally present in the foods like milk, fish, eggs, meat, etc. Most of them are of animal origin and cannot be consumed by vegetarians and vegans. The nowadays vegan population is increasing and because they completely avoid animal products, their diet is usually protein and vitamin-deficient [112]. Therefore, there is a need for an alternate source of supplements that can be adopted by people including the vegan population. Microalgae are photosynthetic organisms with higher growth rates and minimal nutritional requirements. Microalgae remain a prominent source of vitamin D which can be used as a supplement to maintain a balanced diet as well as to resist viral diseases such as COVID-19. Moreover, the well-known source of vitamin D is fish, which acquire it through the food chain from algae due to its inability to de-novo synthesis [113]. Even though microalgae are found to be a prominent producer of vitamin D, their role in microalgal cellular functions is still not clear.
Microalgae are mostly found on stagnant water surfaces and it is assumed that exposure to sunlight, mainly UV-B causes Vitamin D production. Although microalgae are well known as a source of several bioactive compounds, vitamins, and proteins, using microalgae, particularly for vitamin D has been reported very recently [114]. Moreover, studies suggest that exposure of microalgae to UV-B enhances vitamin D production [115].
A recent study conducted in 2020 in which the vitamin D3/cholecalciferol accumulation was assessed under different UVB intensities showed that N. oceanica exhibited the highest vitamin D3 buildup compared to other species studied. They also observed that the safe UVB dose range could vary according to species and going beyond the restricted UVB limit caused further damage to the respective microalgal cells [114,115]. Even though various experiments were conducted to detect Vitamin D production in microalgal species, some of the studies showed the vitamin D3 accumulation to be below the detection limits. A similar work conducted in 1996, examining vitamin D3 production in a mixture of microalgae such as Scenedesmus, Chlorella, Cosmarium, Crucegenia, Oscillatoria, Gomphosphania, Gomphonema, Synedra, Navicula, and Cyclotella indicated somewhat fair amount (~8µg/dry weight) of vitamin D3 accumulation [114,116] According to several other related studies, microalgal species like Tetraselmis suecica (14 µg/g dry weight), Skeletonema costatum (11 µg/g dry weight), Isochrysis galbana (5 µg/g dry weight), and Pavlova lutheri (39 µg/g dry weight) were found to be very prominent Vitamin D producing microalgae, even though some species like Arthrospira maxima, Chlorella minutissima, Rhodomonas salina showed very less vitamin D3 accumulation (less than 0.004 µg/g dry weight) in their cells [114,117,118]. So, microalgae can be cultivated on a large scale to commercialize them as a potent vitamin D supplement. However, increased intake of these vitamin D supplements higher than the recommended dose can result in Vitamin D toxicity which is associated with asymptomatic hypercalcemia leading to the deposition of calcium in kidneys and arterial walls [119,120-127]. Hence, it is advised by health care professionals to intake these supplements not higher than the dosages suggested for respective age groups and physiques.
Most of the clinical data and experimental data demon-strate that vitamin D has a role in lung function and immune response against the foreign pathogen. Some other studies and randomized trials have shown that vitamin D has a protective effect on respiratory tract infections. Vitamin D deficiency may increase the risk of acute respiratory infections and also increase the risk of microbial infections and coronavirus disease. Immune dysregulation is a main feature of coronavirus disease. Their immune balance is important to prevent the hyper-inflammatory cytokine storm in COVID-19 severity and suppress acute respiratory distress syndrome. The role of vitamin D in reducing and modifying the inflammatory cytokine response of respiratory epithelial cells, macrophages, and other immune cells to various pathogens. The antiviral effect of vitamin D which can directly reduce viral replication is dependent on immune modulation and anti-inflammation. Evidently, vitamin D has shown a broad impact on immune cells in both the innate and adaptive immune systems. This is harmonious with the observation that the low status of vitamin D may contrarily impact the outcome of COVID-19 patients. A considerable drop in inflammatory markers and a higher percentage of asymptomatic vitamin D-deficient persons with SARS-CoV-2 infection were both made possible by high dosage, oral vitamin D supplementation to increase 25(OH)D > 50 ng/ml. Cholecalciferol supple-mentation may aid in lowering transmission rates of the extremely contagious SARS-CoV-2 illness by producing SARS-CoV-2 RNA negativity. It will be encouraging to reassure public health professionals about the higher risk of SARS CoV-2 RNA negative in people taking therapeutic cholecalciferol supplements. So, Vitamin D supplementation in corona-risk patients can maintain circulating 25(OH)D levels in the body and microalgae can be one of the potential sources. Moreover, it is relatively inexpensive, safe, and widely available and has also shown propitious effects against viral infections.
This research was supported by the Department of Bioscience, Barkatullah University, Bhopal, Madhya Pradesh, India. A.S would like to thank SERB for the project fellowship and SR would like to thank CSIR for her Ph.D. fellowship. AKB would like to thank SERB, Govt. of India for the SERB-SRG grant. AS, SR, and AKB would like to thank the Department of Microbiology, at the Central University of Tamil Nadu for the administrative support.
- Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014 Mar 20;21(3):319-29. doi: 10.1016/j.chembiol.2013.12.016. Epub 2014 Feb 13. PMID: 24529992; PMCID: PMC3968073.
- Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003 Feb 1;88(2):296-307. doi: 10.1002/jcb.10338. PMID: 12520530.
- Holick MF. Vitamin D: a D-Lightful health perspective. Nutr Rev. 2008 Oct;66(10 Suppl 2):S182-94. doi: 10.1111/j.1753-4887.2008.00104.x. PMID: 18844847.
- Pike JW, Christakos S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol Metab Clin North Am. 2017 Dec;46(4):815-843. doi: 10.1016/j.ecl.2017.07.001. Epub 2017 Sep 29. PMID: 29080638; PMCID: PMC5762112.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007 Jul 19;357(3):266-81. doi: 10.1056/NEJMra070553. PMID: 17634462.
- Ramasamy I. Vitamin D Metabolism and Guidelines for Vitamin D Supplementation. Clin Biochem Rev. 2020 Dec;41(3):103-126. doi: 10.33176/AACB-20-00006. PMID: 33343045; PMCID: PMC7731935.
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009 Feb;19(2):73-8. doi: 10.1016/j.annepidem.2007.12.001. Epub 2008 Mar 10. PMID: 18329892; PMCID: PMC2665033.
- Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011 Jan;86(1):50-60. doi: 10.4065/mcp.2010.0567. PMID: 21193656; PMCID: PMC3012634.
- Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006 Aug;116(8):2062-72. doi: 10.1172/JCI29449. PMID: 16886050; PMCID: PMC1523417.
- Zittermann A, Gummert JF. Nonclassical vitamin D action. Nutrients. 2010 Apr;2(4):408-25. doi: 10.3390/nu2040408. Epub 2010 Mar 25. PMID: 22254030; PMCID: PMC3257656.
- Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017 Jun;18(2):153-165. doi: 10.1007/s11154-017-9424-1. PMID: 28516265.
- Korf H, Decallonne B, Mathieu C. Vitamin D for infections. Curr Opin Endocrinol Diabetes Obes. 2014 Dec;21(6):431-6. doi: 10.1097/MED.0000000000000108. PMID: 25354043.
- Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008 Sep;8(9):685-98. doi: 10.1038/nri2378. PMID: 19172691; PMCID: PMC2906676.
- Aranow C. Vitamin D and the immune system. J Investig Med. 2011 Aug;59(6):881-6. doi: 10.2310/JIM.0b013e31821b8755. PMID: 21527855; PMCID: PMC3166406.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727-733. doi: 10.1056/NEJMoa2001017. Epub 2020 Jan 24. PMID: 31978945; PMCID: PMC7092803.
- Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv Virus Res. 2011;81:85-164. doi: 10.1016/B978-0-12-385885-6.00009-2. PMID: 22094080; PMCID: PMC7149603.
- Harapan H, Itoh N, Yufika A, Winardi W, Keam S, Te H, Megawati D, Hayati Z, Wagner AL, Mudatsir M. Coronavirus disease 2019 (COVID-19): A literature review. J Infect Public Health. 2020 May;13(5):667-673. doi: 10.1016/j.jiph.2020.03.019. Epub 2020 Apr 8. PMID: 32340833; PMCID: PMC7142680.
- Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020 Oct 23;371:m3862. doi: 10.1136/bmj.m3862. PMID: 33097561.
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020 Mar 13;7(1):11. doi: 10.1186/s40779-020-00240-0. PMID: 32169119; PMCID: PMC7068984.
- Ouassou H, Kharchoufa L, Bouhrim M, Daoudi NE, Imtara H, Bencheikh N, ELbouzidi A, Bnouham M. The Pathogenesis of Coronavirus Disease 2019 (COVID-19): Evaluation and Prevention. J Immunol Res. 2020 Jul 10;2020:1357983. doi: 10.1155/2020/1357983. PMID: 32671115; PMCID: PMC7352127.
- Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, Sun C, Sylvia S, Rozelle S, Raat H, Zhou H. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020 Mar 17;9(1):29. doi: 10.1186/s40249-020-00646-x. PMID: 32183901; PMCID: PMC7079521.
- Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J Gen Intern Med. 2020 May;35(5):1545-1549. doi: 10.1007/s11606-020-05762-w. Epub 2020 Mar 4. PMID: 32133578; PMCID: PMC7088708.
- Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, Li P, Zhou Y, Lin YF, Duan Q, Luo G, Fan S, Lu Y, Feng A, Zhan Y, Liang B, Cai W, Zhang L, Du X, Li L, Shu Y, Zou H. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect. 2020 Jun;80(6):656-665. doi: 10.1016/j.jinf.2020.03.041. Epub 2020 Apr 10. PMID: 32283155; PMCID: PMC7151416.
- Cao Z, Li T, Liang L, Wang H, Wei F, Meng S, Cai M, Zhang Y, Xu H, Zhang J, Jin R. Clinical characteristics of Coronavirus Disease 2019 patients in Beijing, China. PLoS One. 2020 Jun 17;15(6):e0234764. doi: 10.1371/journal.pone.0234764. PMID: 32555674; PMCID: PMC7299347.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5. Epub 2020 Jan 24. Erratum in: Lancet. 2020 Jan 30;: PMID: 31986264; PMCID: PMC7159299.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 Mar 17;323(11):1061-1069. doi: 10.1001/jama.2020.1585. Erratum in: JAMA. 2021 Mar 16;325(11):1113. PMID: 32031570; PMCID: PMC7042881.
- McGonagle D, Sharif K, O'Regan A, Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev. 2020 Jun;19(6):102537. doi: 10.1016/j.autrev.2020.102537. Epub 2020 Apr 3. PMID: 32251717; PMCID: PMC7195002.
- Zhong NS, Zheng BJ, Li YM, Poon, Xie ZH, Chan KH, Li PH, Tan SY, Chang Q, Xie JP, Liu XQ, Xu J, Li DX, Yuen KY, Peiris, Guan Y. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003 Oct 25;362(9393):1353-8. doi: 10.1016/s0140-6736(03)14630-2. PMID: 14585636; PMCID: PMC7112415.
- Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020 Apr 2;12(4):988. doi: 10.3390/nu12040988. PMID: 32252338; PMCID: PMC7231123.
- Baraskar K, Thakur P, Shrivastava R, Shrivastava VK. Therapeutic Role of Phytophenol Gallic Acid for the Cure of COVID-19 Pathogenesis. Endocr Metab Immune Disord Drug Targets. 2022 Aug 29. doi: 10.2174/1871530322666220829141401. Epub ahead of print. PMID: 36043737.
- Allegra A, Tonacci A, Pioggia G, Musolino C, Gangemi S. Vitamin deficiency as risk factor for SARS-CoV-2 infection: correlation with susceptibility and prognosis. Eur Rev Med Pharmacol Sci. 2020 Sep;24(18):9721-9738. doi: 10.26355/eurrev_202009_23064. PMID: 33015818.
- Jiménez-Sousa MÁ, Martínez I, Medrano LM, Fernández-Rodríguez A, Resino S. Vitamin D in Human Immunodeficiency Virus Infection: Influence on Immunity and Disease. Front Immunol. 2018 Mar 12;9:458. doi: 10.3389/fimmu.2018.00458. PMID: 29593721; PMCID: PMC5857570.
- Kearns MD, Alvarez JA, Seidel N, Tangpricha V. Impact of vitamin D on infectious disease. Am J Med Sci. 2015 Mar;349(3):245-62. doi: 10.1097/MAJ.0000000000000360. PMID: 25334038; PMCID: PMC4346469.
- Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013 Jul 5;5(7):2502-21. doi: 10.3390/nu5072502. PMID: 23857223; PMCID: PMC3738984..
- Colotta F, Jansson B, Bonelli F. Modulation of inflammatory and immune responses by vitamin D. J Autoimmun. 2017 Dec;85:78-97. doi: 10.1016/j.jaut.2017.07.007. Epub 2017 Jul 18. PMID: 28733125.
- Baeke F, Etten EV, Overbergh L, Mathieu C. Vitamin D3 and the immune system: maintaining the balance in health and disease. Nutr Res Rev. 2007 Jun;20(1):106-18. doi: 10.1017/S0954422407742713. PMID: 19079863
- Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983 Sep 16;221(4616):1181-3. doi: 10.1126/science.6310748. PMID: 6310748.
- Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000 Feb 15;374(2):334-8. doi: 10.1006/abbi.1999.1605. PMID: 10666315.
- Baeke F, Korf H, Overbergh L, van Etten E, Verstuyf A, Gysemans C, Mathieu C. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J Steroid Biochem Mol Biol. 2010 Jul;121(1-2):221-7. doi: 10.1016/j.jsbmb.2010.03.037. Epub 2010 Mar 17. PMID: 20302932.
- Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, Kilby MD, Moss PA, Chakraverty R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003 Jun 1;170(11):5382-90. doi: 10.4049/jimmunol.170.11.5382. PMID: 12759412.
- Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res. 2006 Jan;21(1):37-47. doi: 10.1359/JBMR.050908. Epub 2005 Sep 19. PMID: 16355272.
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006 Mar 24;311(5768):1770-3. doi: 10.1126/science.1123933. Epub 2006 Feb 23. PMID: 16497887.
- Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, Vazirnia A, Zhang X, Steinmeyer A, Zügel U, Hollis BW, Cheng G, Modlin RL. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One. 2009 Jun 5;4(6):e5810. doi: 10.1371/journal.pone.0005810. PMID: 19503839; PMCID: PMC2686169.
- Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004 Sep 1;173(5):2909-12. doi: 10.4049/jimmunol.173.5.2909. Erratum in: J Immunol. 2004 Nov 15;173(10):following 6489. Hanrahan, JH [corrected to Hanrahan, JW]. PMID: 15322146.
- Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K, Sansom DM. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009 Nov 1;183(9):5458-67. doi: 10.4049/jimmunol.0803217. PMID: 19843932; PMCID: PMC2810518.
- Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015 Apr 22;7(4):3011-21. doi: 10.3390/nu7043011. PMID: 25912039; PMCID: PMC4425186.
- Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol. 2009 May;70(5):345-52. doi: 10.1016/j.humimm.2009.01.016. PMID: 19405173..
- Skrobot A, Demkow U, Wachowska M. Immunomodulatory Role of Vitamin D: A Review. Adv Exp Med Biol. 2018;1108:13-23. doi: 10.1007/5584_2018_246. PMID: 30143987.
- Subramanian K, Bergman P, Henriques-Normark B. Vitamin D Promotes Pneumococcal Killing and Modulates Inflammatory Responses in Primary Human Neutrophils. J Innate Immun. 2017;9(4):375-386. doi: 10.1159/000455969. Epub 2017 Feb 28. PMID: 28241127; PMCID: PMC6738809.
- Chirumbolo S, Bjørklund G, Sboarina A, Vella A. The Role of Vitamin D in the Immune System as a Pro-survival Molecule. Clin Ther. 2017 May;39(5):894-916. doi: 10.1016/j.clinthera.2017.03.021. Epub 2017 Apr 21. PMID: 28438353.
- Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010 Aug;10(4):482-96. doi: 10.1016/j.coph.2010.04.001. Epub 2010 Apr 27. PMID: 20427238.
- Bhalla AK, Amento EP, Krane SM. Differential effects of 1,25-dihydroxyvitamin D3 on human lymphocytes and monocyte/macrophages: inhibition of interleukin-2 and augmentation of interleukin-1 production. Cell Immunol. 1986 Apr 1;98(2):311-22. doi: 10.1016/0008-8749(86)90291-1. PMID: 3489547.
- van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005 Oct;97(1-2):93-101. doi: 10.1016/j.jsbmb.2005.06.002. Epub 2005 Jul 19. PMID: 16046118.
- Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007 Aug 1;179(3):1634-47. doi: 10.4049/jimmunol.179.3.1634. PMID: 17641030.
- Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003 Nov 1;102(9):3314-6. doi: 10.1182/blood-2002-11-3521. Epub 2003 Jul 10. PMID: 12855575.
- Müller K, Bendtzen K. 1,25-Dihydroxyvitamin D3 as a natural regulator of human immune functions. J Investig Dermatol Symp Proc. 1996 Apr;1(1):68-71. PMID: 9627696..
- Lemire JM, Adams JS, Kermani-Arab V, Bakke AC, Sakai R, Jordan SC. 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J Immunol. 1985 May;134(5):3032-5. PMID: 3156926..
- van Etten E, Stoffels K, Gysemans C, Mathieu C, Overbergh L. Regulation of vitamin D homeostasis: implications for the immune system. Nutr Rev. 2008 Oct;66(10 Suppl 2):S125-34. doi: 10.1111/j.1753-4887.2008.00096.x. PMID: 18844839.
- Gallo RL, Murakami M, Ohtake T, Zaiou M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J Allergy Clin Immunol. 2002 Dec;110(6):823-31. doi: 10.1067/mai.2002.129801. PMID: 12464945.
- Heine G, Niesner U, Chang HD, Steinmeyer A, Zügel U, Zuberbier T, Radbruch A, Worm M. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur J Immunol. 2008 Aug;38(8):2210-8. doi: 10.1002/eji.200838216. PMID: 18651709.
- Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009 Nov;4(9):1151-65. doi: 10.2217/fmb.09.87. PMID: 19895218; PMCID: PMC2821804.
- Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3). J Cyst Fibros. 2007 Nov 30;6(6):403-10. doi: 10.1016/j.jcf.2007.03.003. Epub 2007 Apr 27. PMID: 17467345; PMCID: PMC2099696.
- Müller K, Diamant M, Bendtzen K. Inhibition of production and function of interleukin-6 by 1,25-dihydroxyvitamin D3. Immunol Lett. 1991 May;28(2):115-20. doi: 10.1016/0165-2478(91)90108-m. PMID: 1885209.
- Hewison M. Vitamin D and innate and adaptive immunity. Vitam Horm. 2011;86:23-62. doi: 10.1016/B978-0-12-386960-9.00002-2. PMID: 21419266.
- Wei R, Christakos S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients. 2015 Sep 24;7(10):8251-60. doi: 10.3390/nu7105392. PMID: 26404359; PMCID: PMC4632412.
- Kongsbak M, Levring TB, Geisler C, von Essen MR. The vitamin d receptor and T cell function. Front Immunol. 2013 Jun 18;4:148. doi: 10.3389/fimmu.2013.00148. PMID: 23785369; PMCID: PMC3684798.
- Bscheider M, Butcher EC. Vitamin D immunoregulation through dendritic cells. Immunology. 2016 Jul;148(3):227-36. doi: 10.1111/imm.12610. PMID: 27040466; PMCID: PMC4913286.
- Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf). 2012 Mar;76(3):315-25. doi: 10.1111/j.1365-2265.2011.04261.x. PMID: 21995874.
- Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, Stepan J, El-Hajj Fuleihan G, Bouillon R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019 Apr;180(4):P23-P54. doi: 10.1530/EJE-18-0736. PMID: 30721133.
- Aparna P, Muthathal S, Nongkynrih B, Gupta SK. Vitamin D deficiency in India. J Family Med Prim Care. 2018 Mar-Apr;7(2):324-330. doi: 10.4103/jfmpc.jfmpc_78_18. PMID: 30090772; PMCID: PMC6060930.
- Kamboj P, Dwivedi S, Toteja GS. Prevalence of hypovitaminosis D in India & way forward. Indian J Med Res. 2018 Nov;148(5):548-556. doi: 10.4103/ijmr.IJMR_1807_18. PMID: 30666982; PMCID: PMC6366270.
- Amrein K, Scherkl M, Hoffmann M, Neuwersch-Sommeregger S, Köstenberger M, Tmava Berisha A, Martucci G, Pilz S, Malle O. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020 Nov;74(11):1498-1513. doi: 10.1038/s41430-020-0558-y. Epub 2020 Jan 20. PMID: 31959942; PMCID: PMC7091696.
- Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J; IOF Committee of Scientific Advisors (CSA) Nutrition Working Group. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009 Nov;20(11):1807-20. doi: 10.1007/s00198-009-0954-6. Epub 2009 Jun 19. Erratum in: Osteoporos Int. 2009 Nov;20(11):1821. PMID: 19543765.
- van Schoor NM, Lips P. Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metab. 2011 Aug;25(4):671-80. doi: 10.1016/j.beem.2011.06.007. PMID: 21872807.
- Martineau AR, Jolliffe DA, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, Grant CC, Janssens W, Jensen ME, Kerley CP, Laaksi I, Manaseki-Holland S, Mauger D, Murdoch DR, Neale R, Rees JR, Simpson S, Stelmach I, Trilok Kumar G, Urashima M, Camargo CA, Griffiths CJ, Hooper RL. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol Assess. 2019 Jan;23(2):1-44. doi: 10.3310/hta23020. PMID: 30675873; PMCID: PMC6369419.
- Dancer RC, Parekh D, Lax S, D'Souza V, Zheng S, Bassford CR, Park D, Bartis DG, Mahida R, Turner AM, Sapey E, Wei W, Naidu B, Stewart PM, Fraser WD, Christopher KB, Cooper MS, Gao F, Sansom DM, Martineau AR, Perkins GD, Thickett DR. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax. 2015 Jul;70(7):617-24. doi: 10.1136/thoraxjnl-2014-206680. Epub 2015 Apr 22. PMID: 25903964; PMCID: PMC4484044.
- Lin R, White JH. The pleiotropic actions of vitamin D. Bioessays. 2004 Jan;26(1):21-8. doi: 10.1002/bies.10368. PMID: 14696037.
- Verstuyf A, Carmeliet G, Bouillon R, Mathieu C. Vitamin D: a pleiotropic hormone. Kidney Int. 2010 Jul;78(2):140-5. doi: 10.1038/ki.2010.17. Epub 2010 Feb 24. PMID: 20182414.
- Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005 Jul;48(7):1247-57. doi: 10.1007/s00125-005-1802-7. Epub 2005 Jun 22. PMID: 15971062.
- Nakashima A, Yokoyama K, Yokoo T, Urashima M. Role of vitamin D in diabetes mellitus and chronic kidney disease. World J Diabetes. 2016 Mar 10;7(5):89-100. doi: 10.4239/wjd.v7.i5.89. PMID: 26981182; PMCID: PMC4781904.
- Legarth C, Grimm D, Wehland M, Bauer J, Krüger M. The Impact of Vitamin D in the Treatment of Essential Hypertension. Int J Mol Sci. 2018 Feb 3;19(2):455. doi: 10.3390/ijms19020455. PMID: 29401665; PMCID: PMC5855677.
- Skowrońska P, Pastuszek E, Kuczyński W, Jaszczoł M, Kuć P, Jakiel G, Wocławek-Potocka I, Łukaszuk K. The role of vitamin D in reproductive dysfunction in women - a systematic review. Ann Agric Environ Med. 2016 Dec 23;23(4):671-676. doi: 10.5604/12321966.1226865. PMID: 28030942.
- Jueraitetibaike K, Ding Z, Wang DD, Peng LP, Jing J, Chen L, Ge X, Qiu XH, Yao B. The effect of vitamin D on sperm motility and the underlying mechanism. Asian J Androl. 2019 Jul-Aug;21(4):400-407. doi: 10.4103/aja.aja_105_18. PMID: 30618415; PMCID: PMC6628736.
- Cito G, Cocci A, Micelli E, Gabutti A, Russo GI, Coccia ME, Franco G, Serni S, Carini M, Natali A. Vitamin D and Male Fertility: An Updated Review. World J Mens Health. 2020 Apr;38(2):164-177. doi: 10.5534/wjmh.190057. Epub 2019 May 17. PMID: 31190482; PMCID: PMC7076312.
- Geleijnse JM. Vitamin D and the prevention of hypertension and cardiovascular diseases: a review of the current evidence. Am J Hypertens. 2011 Mar;24(3):253-62. doi: 10.1038/ajh.2010.199. Epub 2010 Sep 16. PMID: 20847727.
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Apr;8(4):420-422. doi: 10.1016/S2213-2600(20)30076-X. Epub 2020 Feb 18. Erratum in: Lancet Respir Med. 2020 Feb 25;: PMID: 32085846; PMCID: PMC7164771.
- Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, Bucci E, Piacentini M, Ippolito G, Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020 May;27(5):1451-1454. doi: 10.1038/s41418-020-0530-3. Epub 2020 Mar 23. PMID: 32205856; PMCID: PMC7091918.
- Butler-Laporte G, Nakanishi T, Mooser V, Morrison DR, Abdullah T, Adeleye O, Mamlouk N, Kimchi N, Afrasiabi Z, Rezk N, Giliberti A, Renieri A, Chen Y, Zhou S, Forgetta V, Richards JB. Vitamin D and COVID-19 susceptibility and severity in the COVID-19 Host Genetics Initiative: A Mendelian randomization study. PLoS Med. 2021 Jun 1;18(6):e1003605. doi: 10.1371/journal.pmed.1003605. PMID: 34061844; PMCID: PMC8168855.
- Zdrenghea MT, Makrinioti H, Bagacean C, Bush A, Johnston SL, Stanciu LA. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol. 2017 Jan;27(1). doi: 10.1002/rmv.1909. Epub 2016 Oct 7. PMID: 27714929.
- Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol. 2011 Mar;50(3):194-200. doi: 10.1016/j.jcv.2010.12.006. Epub 2011 Jan 15. PMID: 21242105; PMCID: PMC3308600.
- Teymoori-Rad M, Shokri F, Salimi V, Marashi SM. The interplay between vitamin D and viral infections. Rev Med Virol. 2019 Mar;29(2):e2032. doi: 10.1002/rmv.2032. Epub 2019 Jan 6. PMID: 30614127.
- Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010 Jun 14;5(6):e11088. doi: 10.1371/journal.pone.0011088. PMID: 20559424; PMCID: PMC2885414.
- Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. Epidemic influenza and vitamin D. Epidemiol Infect. 2006 Dec;134(6):1129-40. doi: 10.1017/S0950268806007175. Epub 2006 Sep 7. PMID: 16959053; PMCID: PMC2870528.
- Mardani R, Alamdary A, Mousavi Nasab SD, Gholami R, Ahmadi N, Gholami A. Association of vitamin D with the modulation of the disease severity in COVID-19. Virus Res. 2020 Nov;289:198148. doi: 10.1016/j.virusres.2020.198148. Epub 2020 Aug 28. PMID: 32866536; PMCID: PMC7455115.
- Tomaszewska A, Rustecka A, Lipińska-Opałka A, Piprek RP, Kloc M, Kalicki B, Kubiak JZ. The Role of Vitamin D in COVID-19 and the Impact of Pandemic Restrictions on Vitamin D Blood Content. Front Pharmacol. 2022 Feb 21;13:836738. doi: 10.3389/fphar.2022.836738. PMID: 35264968; PMCID: PMC8899722.
- Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID-19 patients. medRxiv. 2020; 2020.04.08. 20058578. https://doi.org/10.1101/2020.04.08.20058578
- Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015; 7(6):4240–4270. https://doi.org/10.3390/nu7064240
- Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020 Jul;32(7):1195-1198. doi: 10.1007/s40520-020-01570-8. Epub 2020 May 6. PMID: 32377965; PMCID: PMC7202265.
- Kohlmeier M. Avoidance of vitamin D deficiency to slow the COVID-19 pandemic. BMJ Nutr Prev Health. 2020 May 20;3(1):67-73. doi: 10.1136/bmjnph-2020-000096. PMID: 33230496; PMCID: PMC7295862.
- DeSmet D, DeSmet K, Herroelen P, Gryspeerdt S, Martens G. Vitamin D deficiency as risk factor for severe COVID-19: a convergence of two pandemics. medRxiv; 2020. https://doi.org/10.1101/2020.05.01.20079376
- Tian Y, Rong L. Letter: Covid-19, and vitamin D. Authors' reply. Aliment Pharmacol Ther. 2020 May;51(10):995-996. doi: 10.1111/apt.15764. PMID: 32286694; PMCID: PMC7262029.
- Arboleda JF, Urcuqui-Inchima S. Vitamin D Supplementation: A Potential Approach for Corona virus/COVID-19 Therapeutics. Front Immunol. 2020; 11:1523. https://doi.org/10.3389/fimmu.2020.01523
- Razdan K, Singh K, Singh D. Vitamin D Levels and COVID-19 Susceptibility: Is there any Correlation? Med Drug Discov. 2020 Sep;7:100051. doi: 10.1016/j.medidd.2020.100051. Epub 2020 Jun 2. PMID: 32835212; PMCID: PMC7266578.
- Weir EK, Thenappan T, Bhargava M, Chen Y. Does vitamin D deficiency increase the severity of COVID-19? Clin Med (Lond). 2020 Jul;20(4):e107-e108. doi: 10.7861/clinmed.2020-0301. Epub 2020 Jun 5. PMID: 32503801; PMCID: PMC7385774.
- Zhang J, McCullough PA, Tecson KM. Vitamin D deficiency in association with endothelial dysfunction: Implications for patients with COVID-19. Rev Cardiovasc Med. 2020 Sep 30;21(3):339-344. doi: 10.31083/j.rcm.2020.03.131. PMID: 33070539.
- Wang L, Lewis T, Zhang YL, Khodier C, Magesh S, Chen L, Inoyama D, Chen Y, Zhen J, Hu L, Beamer LJ, Faloon PW, Dandapani S, Perez JR, Munoz B, Palmer M, Schreiber S. The identification and characterization of non-reactive inhibitor of Keap1-Nrf2 interaction through HTS using a fluorescence polarization assay. 2012 Dec 17 [updated 2013 Sep 16]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 24260785.
- Liu Y, Hyde AS, Simpson MA, Barycki JJ. Emerging regulatory paradigms in glutathione metabolism. Adv Cancer Res. 2014;122:69-101. doi: 10.1016/B978-0-12-420117-0.00002-5. PMID: 24974179; PMCID: PMC4515967.
- Calton EK, Keane KN, Soares MJ, Rowlands J, Newsholme P. Prevailing vitamin D status influences mitochondrial and glycolytic bioenergetics in peripheral blood mononuclear cells obtained from adults. Redox Biol. 2016 Dec;10:243-250. doi: 10.1016/j.redox.2016.10.007. Epub 2016 Oct 20. PMID: 27816874; PMCID: PMC5097975.
- Jäpelt RB, Jakobsen J. Vitamin D in plants: a review of occurrence, analysis, and biosynthesis. Front Plant Sci. 2013 May 13;4:136. doi: 10.3389/fpls.2013.00136. PMID: 23717318; PMCID: PMC3651966.
- Ljubic A, Jacobsen C, Holdt SL, Jakobsen J. Microalgae Nannochloropsis oceanica as a future new natural source of vitamin D3. Food Chem. 2020 Aug 1;320:126627. doi: 10.1016/j.foodchem.2020.126627. Epub 2020 Mar 19. PMID: 32213421.
- Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010 Apr;140(4):817-22. doi: 10.3945/jn.109.118539. Epub 2010 Feb 24. PMID: 20181782; PMCID: PMC2838624.
- Nicoletti M. Microalgae Nutraceuticals. Foods. 2016 Aug 22;5(3):54. doi: 10.3390/foods5030054. PMID: 28231149; PMCID: PMC5302390.
- Del Mondo A, Smerilli A, Sané E, Sansone C, Brunet C. Challenging microalgal vitamins for human health. Microb Cell Fact. 2020 Nov 2;19(1):201. doi: 10.1186/s12934-020-01459-1. PMID: 33138823; PMCID: PMC7607653.
- Ljubic A, Jacobsen C, Holdt SL, Jakobsen J. Microalgae Nannochloropsis oceanica as a future new natural source of vitamin D3. Food Chem. 2020 Aug 1;320:126627. doi: 10.1016/j.foodchem.2020.126627. Epub 2020 Mar 19. PMID: 32213421.
- Ljubic A, Thulesen ET, Jacobsen C, Jakobsen J. UVB exposure stimulates production of vitamin D3 in selected microalgae. Algal Research. 2021; 59: 102472. https://doi.org/10.1016/j.algal.2021.102472.
- Sunita Rao D, Raghuramulu N. Food chain as origin of vitamin D in fish. Comparative Biochemistry and Physiology Part A: Physiology. 1996; 114(1): 15–19. https://doi.org/10.1016/0300-9629(95)02024-1
- de Roeck-Holtzhauer Y, Quere I, Claire C. Vitamin analysis of five planktonic microalgae and one macroalga. Journal of Applied Phycology. 1991; 3(3): 259–264. https://doi.org/10.1007/BF00003584.
- Del Mondo A, Smerilli A, Sané E, Sansone C, Brunet C. Challenging microalgal vitamins for human health. Microb Cell Fact. 2020 Nov 2;19(1):201. doi: 10.1186/s12934-020-01459-1. PMID: 33138823; PMCID: PMC7607653.
- Jäpelt RB, Jakobsen J. Vitamin D in plants: a review of occurrence, analysis, and biosynthesis. Front Plant Sci. 2013 May 13;4:136. doi: 10.3389/fpls.2013.00136. PMID: 23717318; PMCID: PMC3651966.
- Marcinowska-Suchowierska E, Kupisz-Urbańska M, Łukaszkiewicz J, Płudowski P, Jones G. Vitamin D Toxicity-A Clinical Perspective. Front Endocrinol (Lausanne). 2018 Sep 20;9:550. doi: 10.3389/fendo.2018.00550. PMID: 30294301; PMCID: PMC6158375.
- Holick MF, Chen TC. Vitamin D deficiency: a world wide problem with health consequences. Am J ClinNutr. 2008: 87(4):1080S–6S. https://doi.org/10.1093/ajcn/87.4.1080S
- Kuhn JH, Li W, Choe H, Farzan M. Angiotensin-converting enzyme 2: a functional receptor for SARS corona virus. Cell Mol Life Sci. 2004; 2738–2743. https://doi.org/10.1007/s00018-004-4242-5
- Orfanos SE, Armaganidis A, Glynos C, Psevdi E, Kaltsas P, Sarafidou P, Catravas JD, Dafni UG, Langleben D, Roussos C. Pulmonary capillary endothelium-bound angiotensin-converting enzyme activity in acute lunginjury. Circulation. 2000; 102(16): 2011–2018. https://doi.org/10.1161/01.cir.102.16.2011
- Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005 Jul 7;436(7047):112-6. doi: 10.1038/nature03712. PMID: 16001071; PMCID: PMC7094998.
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005 Aug;11(8):875-9. doi: 10.1038/nm1267. Epub 2005 Jul 10. PMID: 16007097; PMCID: PMC7095783.
- Imai Y, Kuba K, Penninger JM. Angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Cell Mol Life Sci. 2007 Aug;64(15):2006-12. doi: 10.1007/s00018-007-6228-6. PMID: 17558469; PMCID: PMC7079778.
- Anita L, Thulesen ET, Jacobsen C, Jakobsen J. UVB Exposure Stimulates Production of Vitamin D 3 in Selected Microalgae. Algal Research. 2021; 59: 102472. https://doi.org/10.1016/j.algal.2021.102472.