More Information
Submitted: January 30, 2024 | Approved: February 20, 2024 | Published: February 21, 2024
How to cite this article: Garuba OD, Anglin JC, Good S, Olufemi SE, Oyawoye OM, et al. Evaluation of Heavy Metals in Commercial Baby Foods. Arch Food Nutr Sci. 2024; 8: 012-020.
DOI: 10.29328/journal.afns.1001056
Copyright License: © 2024 Garuba OD, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Heavy metals; Baby foods; Nutrition; Safe levels; Food system; Minimal risk level; Global contamination
Abbreviations: WHO: World Health Organization; NCDs: Non-communicable diseases; EPA: United States Environmental Protection Agency; ASD: Autism Spectrum Disorder; ATSDR: Agency for Toxic Substances and Disease Registry; UL: Tolerable Upper Intake Level; C2Z: Closer to Zero; HBBF: Healthy Babies Bright Futures
Evaluation of Heavy Metals in Commercial Baby Foods
Omobolanle David Garuba1, Judith C Anglin2, Sonya Good1, Shodimu-Emmanuel Olufemi1, Olubukola Monisola Oyawoye3 and Sodipe Ayodotun1*
1Department of Biology, Department of Chemistry, Texas Southern University, 3100 Cleburne Avenue, Houston Texas 77004, USA
2Department of Health, Nursing and Nutrition, University of District of Columbia, USA
3Department of Microbiology, Federal University, Oye Ekiti, Nigeria
*Address for Correspondence: Sodipe Ayodotun, Ph.D., Department of Biology, Department of Chemistry, Texas Southern University, 3100 Cleburne Avenue, Houston Texas 77004, USA, Email: [email protected]
Nutritious and safe foods are essential to meet normal physiological and metabolic functions. This study evaluated heavy metals in selected food products for newborns and toddlers. These substances may result in adverse health risks and young children are extremely vulnerable due to their immature immune systems and organs. Industrialization and technological advancement have contributed to an increase in heavy metals in the soil; therefore, entering the food system in potentially harmful amounts. Safe levels have been established by monitoring agencies to reduce the presence of heavy metals. Ten national brands of baby foods were analyzed for selected heavy metals. The main ingredients ranged from vegetables, fruits, dairy, poultry, meats, and grains. The products were analyzed in triplicates using QQQ-ICP-MS instrumentation to detect the presence of arsenic, cadmium, zinc, lead, nickel, aluminum, and chromium. Based on the Agency for Toxic Substances and Disease Registry [1] guidelines for safe quantities, aluminum (4.09 µg/g and 2.50 µg/g) and zinc (33.5 µg/g 69.5 µg/g, and 30.2 µg/g) exceeded the recommended levels of 1 µg/g/day and 2 - 3 µg/g /day respectively. Mixed model analysis found significant differences in metal concentrations (F6,24 = 2.75, p = 0.03) with an average metal concentration of 0.96 µg/g. However, no significant correlations were found between the packaging materials used and the observed metal concentrations in the food samples. The study concluded that the presence of heavy metals may be due to food type and the soil on which it is grown and not the packaging materials, establishing food system contamination by heavy metals.
Nutrition is essential for health and development. Adequate and safe nutrition are two important factors that contribute to early childhood optimal growth and proper development [2]. A healthy diet according to the World Health Organization (WHO) helps to protect against not just malnutrition, but also non-communicable diseases (NCDs) such as diabetes, heart disease, and cancer and its likes, therefore healthy dietary practices are encouraged to start early in life to contribute to general wellness [3,4]. Babies and infants have underdeveloped organs and immune systems and are easily vulnerable to illnesses and toxins, therefore extreme care should be taken to ensure the safety and adequacy of foods. During the first six months of life, according to the WHO recommendation, infants are to be exclusively breastfed and thereafter be introduced to foods that complementarily support their growth and development [5].
Exclusive breastfeeding promotes and improves cognitive development although not every child is privileged to benefit from this because many babies start off on infant formulas due to demanding unavoidable situations such as maternal death, premature delivery, low birth weight, and in most cases, the convenience of usage since any caregiver can help feed the baby as opposed to just the mother, especially due to the demands of work [2].
Foods that are tailored to partially or fully meet the nutritional needs of children from newborns to toddlers (3 years) are frequently referred to as baby foods. Food processing includes cooking, heat treatments, and the addition of additives to improve the taste, color, texture, and shelf-life of the product. Chemical and physical reactions caused by processing may however introduce harmful by-products into the food [5]. Also, toxic substances and heavy metals such as cadmium, arsenic, lead, and mercury are often found in food, including baby foods as a result of their absorption by crops from soil and water during the planting period [6,7], and in most cases, through migration from packaging materials such as ‘cans’ used during the packaging process and storage time [8-11].
Although heavy metals such as lead (Pb), cadmium (Cd), mercury (Hg), and arsenic (As) have no known functionality or nutritional values in the human body, these metals have many industrial uses such as in electroplating, and in making battery components and circuit boards that are used in modern electronics. If found in the human body, they are due to exposure to sources such as groundwater, contaminated food products, forest fire, volcanic ash, natural erosion, factory, and power plant emissions, regulated release by industries into the natural waterways, and also through unregulated pollution. Consequentially, when these elements make their way into the food chain, they are found in detectable quantities in all commercially available food products, including baby foods [6,12].
Although the scientific community widely uses the term “heavy metals,” there is no standardized authoritative definition describing this term [13,14]. Several heavy metals are elements that are essential soil and plant nutrients and may be required by the human body in minute amounts. The Food and Nutrition Board of the National Academies of Sciences Engineering, and Medicine has established a Tolerable Upper Intake Level (UL) of daily maximum intake without adverse effects. For example, Chromium (Cr) and Nickel (Ni) are essential for human health in minor quantities, playing important roles in cellular activities [15-17]. High dietary intake of heavy metals affects all age groups; however, children are at higher risk [18]. From six months of age, the Dietary Guidelines for Americans [19] recommends introducing foods other than breastmilk and infant formula. Of these categories of foods, cereals, fruits, and vegetables contribute the most dietary exposure to heavy metals in children and when they sometimes exceed the UL, they accumulate in the body due to babies’ underdeveloped organ’s ability to eliminate them efficiently [20-22].
Metal toxicity therefore depends on factors such as physicochemical properties, amount ingested and frequency of ingestion, individual health status, and their synergistic and antagonistic properties due to the presence of other chemical compounds [23,24]. The levels must be maintained within a safe range to prevent nutritional deficiencies as well as the health concerns associated with higher concentrations and bioaccumulation in cells [17]. The FDA has limited guidance on heavy metal concentrations in food products consumed by children and especially, infants, however, some metals such as cadmium do not have a clearly established permissible limit, while mercury levels have been restricted to fish and fish products alone [25,26]. Due to emerging issues of food safety across the globe, and the inextricable associations linked to human health, the United States Environmental Protection Agency (EPA) and the Agency for Toxic Substances and Disease Registry [27] have included arsenic (As), lead (Pb), cadmium (Cd) and (mercury) Hg in the top twenty list of dangerous substances and have presented the minimal risk levels which are considered safe and within the acceptable threshold limit for those metals [28-33].
Excessive heavy metal food contamination is a global phenomenon posing serious health risks and perturbing the ecosystem [17,22,23,34,35]. Although heavy metals occur naturally in the environment, they are also released anthropogenically into the environment as industrial pollutants [36-38], thereby finding their way into the food chain through plant uptake, livestock consumption of contaminated water or food and/or agricultural as well as manufacturing processes [14,36]. The conflicting reports associating heavy metal intoxication with Autism Spectrum Disorders (ASD) as well as attention deficit disorder (ADHD) remain to be explored [39]. Since the etiology of ASD remains largely unknown, possible causes are linked but not limited to environmental factors such as ingestion of heavy metal-contaminated foods either maternally prior to pregnancy or through direct ingestion of contaminated baby foods [40-42]. Several studies over the years have shown toxic chemicals and heavy metals above the UL in baby foods across the globe [14,17,43-45] increasing concerns on their potential adverse effects.
To ensure safety, the U.S. Food and Drug Administration (FDA) regulates the heavy metal content of food products [46]. Additionally, non-governmental organizations such as the Consumer Reports and Healthy Babies Bright Futures (HBBF), especially since 2017 have been more committed to ensuring that food products, especially baby, toddler, and adolescent food products are tested for the presence of heavy metals [14]. The EPA works jointly with the Agency for Toxic Substances and Disease Registry to review, respond, and monitor the health threats associated with known heavy metals [25]. In 2021, the US FDA issued the Closer to Zero (C2Z) action plan for foods eaten by babies and young children to monitor and reduce toxic element exposure to the lowest possible concentrations through food in order to protect the pediatric population [47].
The purpose of this study is to evaluate heavy metal contents in selected food products for newborns to toddler age groups.
Collection of samples
For this study, the inclusion criteria are processed foods for infants and toddlers between the ages of 6 months to 3 years, containing one or more of the following ingredients: dairy, fruits, vegetables, root vegetables, grains, sugar supplements, and juice. The selected products are available in multi-chain retail stores throughout the country. Ten (10) different food products were purchased in triplicates from a local store in Houston, Texas. These included two brands of infant formulas and stages 2 and 3 baby food puree labeled “organic”. The other six brands were labeled “natural”. Product packing materials were either plastic tubs, glass jars, or aluminum pouches and cans. The products ranged from a single type of fruit or vegetable or a mixture of 2 or 3 items from the ingredients identified earlier. All samples had valid expiration dates to ensure product stability and quality. Samples were analyzed in triplicates to identify variability in heavy metal concentrations with each product type.
Table 1 provides details on each sample including the ingredient categorization, brand, food labelling (organic and natural), and packaging material.
| Table 1: Food sample types and consumer age group category. | |||||
| Sample ID | Primary Ingredient(s) | Food Category | Packaging Material | Size | Consumer Age Group |
| S1 Brand1 |
Sweet potato and turkey puree | Natural | Squeezable pouch | 3.5 oz | Stage 3: 6 - 12 months |
| S2 Brand 2 |
Banana and raspberry fruities | Natural | Squeezable pouch |
3.5 oz | Stage 2: 6 - 9 months |
| S3 Brand 3 |
Sweet potato and apple puree | Organic Non - GMO |
Squeezable pouch | 4 oz | Stage 3: 6 - 12 months |
| S4 Brand 4 |
Chicken casserole, vegetable, and rice puree | Organic non - GMO | Squeezable pouch | 4.5 oz | Stage 3: 6 - 12 months |
| S5 Brand 3 |
Rice cereal | Natural | Plastic | 16 oz | Stage 2 supported sitter 4 - 6 months |
| S6 Brand 5 |
Syrup | Natural | Plastic | 8 oz | Toddlers 2 - 6+ years |
| S7 Brand 5 |
Beef and gravy | Natural | Glass jar | 2.5 oz | Sitter: 6 - 8 months |
| S8 Brand 6 |
Apple, orange, strawberry, and cherry fruit punch | Natural | Box pouch | 6 Fl/oz | Toddlers 2 - 6+ years |
| S9 Brand7 |
Infant formula A | Natural non - GMO | White vanish lined Aluminum can | 12.7 oz | Stage 1: 0 - 12 months |
| S10 Brand 5 |
Infant formula B | Natural non - GMO | White vanish lined Aluminum can | 12.4 oz | Stage 1: 0 - 12 months |
Statistical analysis
Heavy metal values are expressed as mean ± SEM of triplicate measurement. Analysis of variance was performed to compare the mean differences among the groups (food types) using (SAS, 2004). Regression analysis was also performed to model the relationship between heavy metal concentrations and the packaging materials used for the different food products. In all cases, a p - value greater than 0.05 was considered insignificant.
Sample and standards preparation
The food samples were sent in the original, sealed packaging to the ICP Laboratory in the Center for Advanced Analytical Geochemistry (CAAG) at the University of Houston, Houston, Texas, and analyzed in triplicates using the Single Reaction Chamber (SRC) Microwave digestion method. This system operates at a maximum power of 1500 W (Table 2). Each sample of 500 mg was placed into 40 ml quartz tubes, and 5 ml of 16 N HNO3 + 1 ml of 12 N HCl + 1 ml of 30% H2O2 were added into each tube. The tubes were loaded into the SRC microwave system for digestion. After digestion, the solutions were transferred and comingled into a single PFA beaker and dried down incipiently on the hot plate at about 180 oC. 2% HNO3 was added into the beaker and heated for 30 min - 90 min at about 180 oC to assure re-dissolve. The sample solution was diluted to about 4-gram solution at a low dilution factor (≈10x) to form the final solution. Samples were then analyzed for total Al, Cr, As, Cd, Zn, and Pb with the exception of Hg using the triple quadrupole ICP-MS (QQQ-ICP-MS) method according to Milestone’s UltraWAVE method [48]. This method, inductively coupled plasma optical emission spectrometers, uses an additional quadrupole mass filter before collision/reaction cell (CRC) which resolves spectral interferences and overlaps while filtering the ions twice and ensuring a 95% recovery rate as described by Yang, et al. [49].
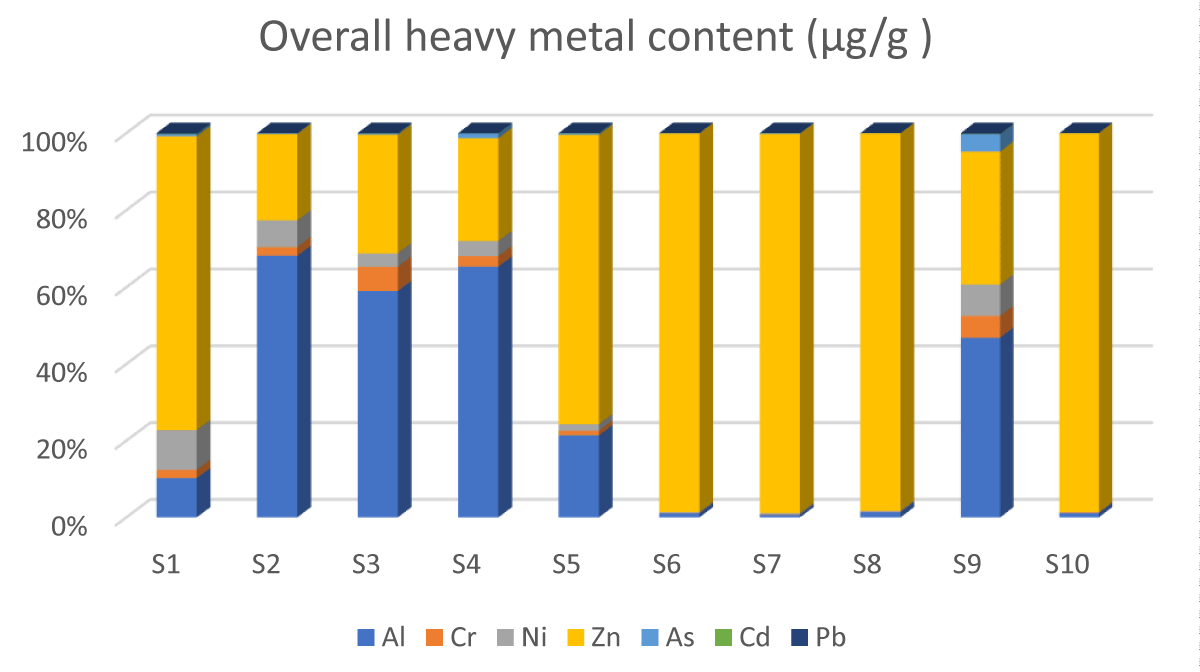
Table 1 provides information about the food type, food category, and packaging materials used for the different food products. The food categories were classified as being organic, natural, or genetically/non-genetically modified products, while the packaging materials varied between gas jars, vanish lined aluminum pouches, laminated squeezable pouches, plastic, and even paper boxes. The result from this study shows that natural, organic, and non-genetically modified food products all tested positive for evaluated heavy metals at varying concentrations. Based on the regression analysis model used, it is evident that the presence of heavy metals in all evaluated food samples was not a result of cross-migration from the different packaging materials used. Table 2 shows the operating condition of the QQQ-ICPMS used to detect the various metals, establishing the effectiveness of this technique in detecting analytes even at low detection limits. Heavy metal concentration values are expressed as mean and standard deviation of triplicate measurements as seen in Table 3. Mixed model analysis of variance using SAS, revealed significant differences in metal concentrations among the food types ((F6,24 = 2.75, p = 0.035)) as presented in Table 4. This shows that the presence of heavy metals in varying concentrations was independent of the brand since heavy metals were detected in all seven brands of baby foods although regression analysis revealed no significant differences between the metal concentration and the packaging materials used as presented in Table 5. In all cases, a p - value greater than 0.05 was considered not statistically significant. The minimal risk level values used as the standard to compare established safe exposure limits metal values are presented in Table 6, which is an updated version according to the FDA. The overall heavy metal concentrations in all evaluated food types are presented in Figure 1.
Figure 1: Comparative description of the overall metal content in all food types./p>
| Table 2: Operating conditions of the QQQ-ICP-MS UltraWAVE SRC system. | |
| Parameter | Setting |
| RF Power | 1500 W |
| Temperature range | 90 oC min - 300 oC max |
| Pressure range | 15 MPa to 19.9 MPa |
| PTFE liner material | Pure Nitrogen (N2) |
| Nebulizer | Concentric |
| Plasma gas flow rate (L/min) | 1.8 |
| Carrier gas (L/min) | 1.07 |
| Table 3: Mean value concentrations of the heavy metal contents of the 10 food types. | |||||||
| Food type | (Al) µg/g mean (SD) | (Cr) µg/g mean (SD) | (Ni) µg/g mean (SD) | (Zn) µg/g mean (SD) | (As) µg/g mean (SD) | (Cd) µg/g mean (SD) | (Pb) µg/g mean (SD) |
| S1 | 0.217 (0.06) |
0.045 (0.012) |
0.220 (0.202) |
1.617 (0.014) |
0.011 (0.001) |
0.003 (0.000) |
0.004 (0.001) |
| S2 | 4.089 (0.172) |
0.136 (0.098) |
0.414 (0.300) |
1.346 (0.024) |
0.012 (0.001) |
0.001 (0.000) |
0.001 (0.000) |
| S3 | 2.501 (0.355) |
0.267 (0.046) |
0.148 (0.004) |
1.304 (0.092) |
0.013 (0.001) |
0.002 (0.001) |
0.003 (0.000) |
| S4 | 0.316 (0.048) |
0.013 (0.003) |
0.019 (0.012) |
0.006 (0.001) |
0.006 (0.001) |
0.000 (0.000) |
0.000 (0.000) |
| S5 | 0.824 (0.060) |
0.048 (0.005) |
0.065 (0.006) |
2.9000 (0.006) |
0.013 (0.001) |
0.004 (0.001) |
0.001 (0.000) |
| S6 | 0.353 (0.000) |
0.032 (0.001) |
0.095 (0.001) |
33.553 (1.569) |
0.023 (0.001) |
0.000 (0.000) |
0.002 (0.000) |
| S7 | 0.492 (0.038) |
0.067 (0.008) |
0.235 (0.011) |
69.535 (3.198) |
0.102 (0.004) |
0.017 (0.001) |
0.001 (0.000) |
| S8 | 0.416 (0.034) |
0.037 (0.013) |
0.069 (0.016) |
30.247 (1.497) |
0.012 (0.001) |
0.000 (0.000) |
0.0007 |
| S9 | 0.085 (0.023) |
0.010 (0.003) |
0.015 (0.002) |
0.063 (0.020) |
0.008 (0.000) |
0.000 (0.000) |
0.000 (0.000) |
| S10 | 0.271 (0.023) |
0.023 (0.008) |
0.020 (0.001) |
23.694 (1.346) |
0.009 (0.001) |
0.000 (0.000) |
0.001 (0.000) |
|
|||||||
| Table 4: ANOVA analysis showing the difference in metal concentrations in all food types. | ||||||
| Term | Estimate | Standard error | Statistic | DF | p - value | Main effect |
| (Intercept) | 0.959134 | 0.392284 | 2.445003 | 10.04282 | 0.034457 | Intercept |
| Metal Pb | -0.95801 | 0.400979 | -2.38918 | 10.13393 | 0.037705 | Lead |
| Metal Cd | -0.95733 | 0.401171 | -2.38633 | 10.13392 | 0.03789 | Cadmium |
| Metal As | -0.94034 | 0.402094 | -2.33861 | 10.134 | 0.041121 | Arsenic |
| Metal Cr | -0.89432 | 0.384381 | -2.32665 | 10.15306 | 0.041927 | Chromium |
| Metal Ni | -0.83424 | 0.373711 | -2.23231 | 10.16474 | 0.049234 | Nickel |
| Metal Zn | 15.48043 | 7.009124 | 2.208612 | 10.02066 | 0.051629 | Zinc |
This study uses the Minimal Risk Level values (MRLs) of the Agency for Toxic Substances and Disease Registry (ASTDR) which is developed jointly with the U.S. Environmental Protection Agency (EPA) and is based on the toxicological profiles of the metals of interest as seen in Table 6 [50] to determine increased health risks. Based on the findings of this study, aluminum and zinc had the highest concentration in the products as seen in Figure 1. The highest concentrations of aluminum were observed in food samples S2 and S3, (4.09 µg/g and 2.50 µg/g) which are stages 2 and 3 food categories, providing nourishment for infants within the 6 months - 9 months age group. Descriptively, these two food types are plant-based products which could explain why they have elevated concentrations of the observed metals. This may be a result of the food chain contamination during the planting process since aluminum is very abundant in the earth’s crust. Food samples S2 and S3 exceed the oral MRL of aluminum which is set at 1 µg/g a day, considering that these concentrations were observed per 500 mg of each food product. Although both food products were packaged in squeezable pouches of similar packaging material and produced by different manufacturers, no significant differences were found in the metal concentrations based on the packaging material used (p = 0.63, std error 1.94). A previous study by Chuchu, et al. [51] observed elevated levels of aluminum in infant formulas, attributing this to the soy content of dairy products. This study, however, observed lower levels in similar food products at levels below the MRL. Aluminum is considered ubiquitous because it is the third most abundant element in the earth’s crust, being unavoidably present in tap water, evaporated salt, white sugar, and in most ingredients, such as flour, and baking powder used in making cookies for our children. There is no evidence from previous studies that children are more sensitive to aluminum than adults, but it has been linked to kidney problems when its concentration exceeds the tolerable level [51].
Zinc concentration was the second highest found in the samples. Zinc is naturally found in grains, meat, and meat products. Zinc is often added to cereals and dairy products for fortification, and to dietary supplements [52]. This element is ubiquitous and is an essential micromineral helping to maintain and balance homeostasis. Zinc is a component of several metalloenzymes that are needed for DNA synthesis. The Dietary Reference Intakes developed by the Food and Nutrition Board of the National Academies of Sciences Engineering, and Medicine set the recommended daily intake of zinc at 2 mg daily for children aged 0 months – 6 months and 3 mg from 7 months to 3 years. Also, the National Institute of Health (NIH) set the MRL of zinc for infants and toddlers at 2 - 3 µg/g daily while the WHO/FAO set the upper limit intake at 7 µg/g /day [53,54]. The study results found levels higher than the recommended amount in samples S6 (an infant formula) at 33.5 µg/g, S7 (rice cereal) at 69.5 µg/g, and S8 (an infant formula) at 30.2 µg/g. as seen in Table 3. Excessive oral intake of zinc is rarely associated with toxicity; however, some studies have documented immunosuppressive effects that could promote pathogen multiplication, diarrhea, stomach pain, nausea, and vomiting [52,55].
Chromium was also detected in all the evaluated food types, although the highest levels were recorded in S2 at 0.13 ug/g (pear, banana, and raspberry fruities) and S3 at 0.27 ug/g (sweet potato and apple puree) which is an organic food product as seen in Table 3. The minimal proposed limit level for chromium is 0.1 ug/g and these two-plant product-formulated food samples slightly exceeded this limit. Chromium has been described to be an essential trace element that can improve insulin sensitivity and enhance metabolism. Its deficiency includes weight loss, and reduced response to glucose in the blood, which increases the risk of diabetes along with many other factors in the body. Its abundance is usually observed in sweet potatoes and broccoli [56] agreeing with the findings from this study. The lowest levels were observed in S9 (syrup) and S4 (Caprisun) which are mostly non-plant-based sugar products, serving children in the 1–2-year age groups.
Plant-based product samples had the highest nickel content. These are rice cereal (S7), sweet potato, apple mix (S3), pear, banana, and raspberries (S2), and sweet potato and turkey puree (S1). The concentration values were between 0.14 µg/g and -0.41 µg/g. Although nickel levels are typically low in the environment, its use in modern technology such as in stainless steel, artillery, electrical equipment, electroplating, mordant of dye, and domestic utensils is gradually increasing its widespread release and hyper-accumulation in various locations of the earth such as seawater, ashes of marine plants, sewage sludge, and agricultural soils [57,58]. Nickel increase in the environment is clearly linked to anthropogenic activities. Although nickel is an essential micronutrient, being a component of enzymes needed for iron absorption [59], the associated adverse effects of excessive intake of nickel are linked to genotoxicity, hematotoxicity, and neurotoxicity which have all been documented [57,60].
Lead and cadmium levels were low and none of the food types evaluated exceeded the recommended permissible limit as seen in Table 2. The highest concentrations of lead in this study were observed in S1 and S3, both of which contained sweet potato. This finding is consistent with Parker, et al. [14,61], and Gu, et al. [51]. Sweet potatoes grow underground and lead naturally occurs in small quantities in the earth’s crust. Cadmium on the other hand was elevated the most in S7 which is rice cereal. This finding corroborates with previous studies [14,29,62] which indicated that rice is a major source of cadmium. A study by Rahimzadeh, et al. [63] revealed that cadmium abundance in the environment is a result of human activities such as the use of fossil fuel, waste burning, and sewage leaking sludge to agricultural soils, which may cause the transfer of cadmium compounds absorbed by plants through food chain contamination to final accumulation in various human organs.
Arsenic levels were low in all food types except in S7 which is rice cereal (0.1 ug/g). Similar levels were reported previously by Alharbi, et al. [64]. However, the slightly elevated arsenic level observed in rice cereal agrees with Parker, et al. [14] who reported the highest levels of arsenic in rice cereal in their study. The MRL of arsenic is dependent on its organic form. For example, the MRL of Monomethyl Arsenic Acid (MMA) is 0.00001 ug/g/day while inorganic arsenic is aet at 0.005 ug/g/day. Presently, the FDA only regulates arsenic in infant rice cereal at a high level of 0.1 ug/g which clearly shows that the total arsenic concentrations in this study were generally low although the endpoint of its toxicity will depend primarily on its organic state. Arsenic is naturally present in groundwater and is highly toxic. Rice plants are naturally semi-aquatic, and arsenic is absorbed from groundwater [65]. Also, the use of arsenic in agricultural planting processes despite its toxicity remains a major source of arsenic in infant foods to date [19,66].
Overall, there were statistically significant differences in the heavy metal concentrations among the samples (Table 4) indicating that heavy metal concentration was not based on food brand but on food type. It is important to note that the heavy metal concentration did not exceed the MRLs except for aluminum and zinc. This study supports the 2021 report by the FDA and the need for continuous monitoring. This study also shows no statistically significant differences between metal concentrations and packaging materials used for the food products (Table 5), corroborating with findings by Munro, et al. [67-76]. The study provides further evidence that heavy metals in baby foods were not a result of migration from packaging materials to food products (Table 1).
Heavy metal toxicity remains a threat to human health because of the risks associated with its chronic bio-cumulative potential. Anthropogenic activities continue to contribute to the increasing levels of these metals in our environment. Based on the findings from this study, it is evident that soil-to-crop contamination contributes significantly to increasing metal concentrations in food products. It is noteworthy that there was no significant correlation establishing metal migration from packaging materials in any of the evaluated food brands. This indicates an improvement in the quality of packaging materials used in packing and storing baby foods. Although most of the metals evaluated in this study were below the proposed MRL standard used, low metal detection is not equivalent to no adverse effects. Some heavy metals like arsenic and cadmium are potentially toxic even at extremely low concentrations. Heavy metal toxicity depends largely on the amount ingested, frequency of ingestion, bio-accumulation, and detoxification amidst individual factors such as health status, age, and genetic makeup. Infants and toddlers are a vulnerable population, whose frequency of ingestion of metals may be higher since the convenience of usage of these food products by parents may increase their daily intakes above daily minimal risk levels. It is concerning that all evaluated food products had detectable metal levels, signifying that a wide variety of baby food products are potential dietary exposure routes to children. Therefore, continuous strict monitoring is required to ensure that toxic metal levels are always kept below levels to minimize adverse effects.
Plant-based food products exhibited the highest contaminations. This study provides additional information about the importance of safe food systems and reducing harmful compounds from entering the system. Where and how food is grown is critical to reducing contamination of food supplies and systems from anthropogenic sources and to helping farmers improve agricultural practices such as the use of contaminated soil, water, and industrial discharge/effluents on agricultural lands. Monitoring food and ensuring food safety effectively depends on the combined efforts of federal agencies such as the FDA and the United States Department of Agriculture (USDA), farmers, food producers/manufacturers, food suppliers, and researchers to contribute evidence that may be used to develop guidelines and monitoring tools. The Closer to Zero (C2Z) plan initiated in 2021 by the FDA may become increasingly helpful with this and other studies providing additional scientific data to fully implement the plan.
Funding
This work was funded by the Research and Innovation Center, Texas Southern University, Houston, Texas.
Data and material availability
The data and materials used in this research can be made available upon request.
Authors’ contributions
All authors listed in this article have contributed significantly to the research, writing, and editing of this manuscript.
Consent for publication: All authors have provided consent for the publication of this article.
- ATSDR. Toxicological profile for Arsenic. 2007. https://www.atsdr.cdc.gov/toxprofiles/tp2.pdf
- Ibrahim C, Kammouni Z, Barake M, Kassir M, Al-Jawaldeh A, Matta J, Sacre Y, Hanna-Wakim L, Haddad J, Hoteit M. Pediatric Health Risk Assessment for Exposure to Aluminum from Infant Formulas and Children under the Age of Five's Food Products among Arab Infants: Experience from Lebanon. Foods. 2022 Aug 19;11(16):2503. doi: 10.3390/foods11162503. PMID: 36010503; PMCID: PMC9407326.
- World Health Organization (WHO): Healthy diet 29 April 2020 https://www.who.int/news-room/fact-sheets/detail/healthy-diet
- FDA. Lead in Food, Food wares, and Dietary Supplements. 2018a. https://www.fda.gov/food/metals/lead-food-foodwares-and-dietary-supplements
- Rafaela P, Petrarca MH, Filho JT, Goday HT. Simultaneous determination of furfural, 5-hydroxymethylfurfural and 4-hydroxy-2,5-dimethyl-3 (2H)- furanone in baby foods available in the Brazilian market, Journal of Food Composition and Analysis. 2021; 99:103874.
- Sam A, Robert RC. Evaluation of stage 2 baby foods as potential source of heavy metal toxicity in infants 6 to 12 months. Journal of Undergraduate Chemistry Research. 2021; 20(2):23. doi: 10.3389/fnut.2022.919913
- Saeed S, Amir J, Siroos S, Yeboah GA and Jesus RC. Heavy metal uptake by plants from wastewater of different pulp concentrations and contaminated soils. Journal of cleaner production. 2021; 286:126345, https://doi.org/10.1016/j.jclepro.2021.126345.
- Eleboudy AA, Amer AA, El-Makarem A, Abo HS, Hadour HH. Heavy metal residues in some dairy products. Alexandria Journal of Veterinary Sciences (AJVS). 2016; 52:1; 334-346. https://doi.org/10.1016/j.focha.2023.100261
- Guhur ME, Gamze C. Toxic metals in paper and paperboard food packaging. BioResources. 2018; 13(4): 7560-7580.
- Joon-Goo L, Jeong-Yun H, Hye-Eun L, Tae-Hun K, Jang-Duck C, Gil-Jin G. Effects of food processing methods on migration of heavy metals to food. Appl. Biol Chem. 2019; 6264:1-10. https://doi.org/10.1186/s13765-019-0470-0.
- El-Shaer M, El-Kholie EA, Abdelah SH. Migration of Iron and some toxic metals to foodstuffs during storage. Journal of Home Economics. 2022; 32(2):64-79. DOI: 10.21608/MKAS.2022.113347.1104
- Wong C, Roberts SM, Saab IN. Review of regulatory reference values and background levels for heavy metals in the human diet. Regul Toxicol Pharmacol. 2022 Apr; 130:105122. doi: 10.1016/j.yrtph.2022.105122. Epub 2022 Jan 26. PMID: 35090957.
- Ali H, Khan E. What are heavy metals? long-standing controversy over the scientific use of the term ‘heavy metals’—proposal of a comprehensive definition, Toxicological & Environmental Chemistry. 2017. http://dx.doi.org/10.1080/02772248.2017.1413652
- Parker GH, Gillie CE, Miller JV, Badger DE, Kreider ML. Human health risk assessment of arsenic, cadmium, lead, and mercury ingestion from baby foods. Toxicol Rep. 2022 Feb 4; 9:238-249. doi: 10.1016/j.toxrep.2022.02.001. PMID: 35198407; PMCID: PMC8850323.
- EFSA. Scientific opinion on dietary reference values for chromium. European Food Safety Authority (EFSA) Eur. Food Safety Authority (EFSA). 2013; 12 (10):25.
- Health Canada. Guidelines for Canadian Drinking Water Quality – Chromium. 2018. www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-
- Naveed M, Jahangeer M, Bouyahya A, Omari NEl, Ghchime R, Balahbib A, Aboulaghras S, Mahmood Z, Akram M, Muhammad S, Shah A, Ivan NM, Marina D, Rebezov M, Venkidasamy B, Thiruvengadam M, Ali Shariat M: Heavy metal contamination of natural foods is a serious health issue: a review. Sustainability. 2022; 14:161. https://doi.org/10.3390/su14010161
- Vogt R, Bennett D, Cassady D, Frost J, Ritz B, Hertz-Picciotto I. Cancer and non-cancer health effects from food contaminant exposures for children and adults in California: a risk assessment. Environ Health. 2012 Nov 9; 11:83. doi: 10.1186/1476-069X-11-83. PMID: 23140444; PMCID: PMC3551655.
- Dietary Guidelines for Americans. health.gov 2020-2025
- Dominiquez A, Paz S, Rubio C, Gutierrez A, Gonzalez-Weller D, Revert C, Hardisson Arturo. Essential and toxic metals in infant formula from the European community. Open Acc. J of Toxicol. 2017; 2:2. DOI:10.19080/OAJT.2017.02.555585
- Moein B, Ahansaz A, Mahshid B, Amiri B, Nasiri AK. Prevalence of heavy metals in cereal-based baby foods: protocol of a systematic review study. 2021. DOI:10.13140/RG.2.2.23088.89602
- Shah J, Said M, Wajid A, Luqman HM. Potential risks assessment of heavy metal(loids) contaminated vegetables in Pakistan: a review. Geocarto International. 2021; 37:24. https://doi.org/10.1080/10106049.2021.1969449
- Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014 Jun;7(2):60-72. doi: 10.2478/intox-2014-0009. Epub 2014 Nov 15. PMID: 26109881; PMCID: PMC4427717.
- de Almeida CC, Baião DDS, Rodrigues PA, Saint'Pierre TD, Hauser-Davis RA, Leandro KC, Paschoalin VMF, da Costa MP, Conte-Junior CA. Toxic Metals and Metalloids in Infant Formulas Marketed in Brazil, and Child Health Risks According to the Target Hazard Quotients and Target Cancer Risk. Int J Environ Res Public Health. 2022 Sep 6;19(18):11178. doi: 10.3390/ijerph191811178. PMID: 36141460; PMCID: PMC9517614.
- FDA: Total Diet Study 2018. https://www.fda.gov/food/science-research-food/total-diet-study
- Amarh FA, Agorku ES, Voegborlo RB, Ashong GW, Atongo GA. Health risk assessment of some selected heavy metals in infant food sold in Wa, Ghana. Heliyon. 2023 May 12;9(5):e16225. doi: 10.1016/j.heliyon.2023.e16225. PMID: 37215839; PMCID: PMC10196951.
- ATSDR. Toxicological Profile for Barium. U.S. Department of Health and Human Services, Public Health Service, Atlanta. GA. 2007.
- ATSDR. Toxicological profile for Arsenic. 2007. https://www.atsdr.cdc.gov/toxprofiles/tp2.pdf
- Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Exp Suppl. 2012; 101:133-64. doi: 10.1007/978-3-7643-8340-4_6. PMID: 22945569; PMCID: PMC4144270.
- Xiong T, Austruy A, Pierart A, Shahid M, Schreck E, Mombo S, Dumat C. Kinetic study of phytotoxicity induced by foliar lead uptake for vegetables exposed to fine particles and implications for sustainable urban agriculture. J Environ Sci (China). 2016 Aug; 46:16-27. doi: 10.1016/j.jes.2015.08.029. Epub 2016 Feb 3. PMID: 27521932.
- Xiong T, Dumat C, Pierart A, Shahid M, Kang Y, Li N, Bertoni G, Laplanche C. Measurement of metal bioaccessibility in vegetables to improve human exposure assessments: field study of soil-plant-atmosphere transfers in urban areas, South China. Environ Geochem Health. 2016 Dec;38(6):1283-1301. doi: 10.1007/s10653-016-9796-2. Epub 2016 Jan 29. PMID: 26825060.
- Sana K, Muhammad S, Khan NN, Behzad M, Irshad B, Camille D, Geochem J. A comparison of technologies for remediation of heavy metal contaminated soils. Explor. 2017; 182 (B):247–268. https://doi.org/10.1016/j.gexplo.2016.11.021
- Rai PK. Phytoremediation of Emerging Contaminants in Wetlands. Taylor & Francis, Boca Raton, Florida, USA. 2018; 248. https://doi.org/10.1201/9781351067430
- Said M, Kashif A. Heavy metal contamination in water and fish of the Hunza River and its tributaries in Gilgit-Baltistan: Evaluation of potential risks and provenance. Environmental Technology and Innovation. 2020; 20: 101159. https://doi.org/10.1016/j.eti.2020.101159
- Muhammad S. Evaluation of heavy metals in water and sediments, pollution, and risk indices of Naltar Lakes, Pakistan. Environ Sci Pollut Res Int. 2023 Feb;30(10):28217-28226. doi: 10.1007/s11356-022-24160-9. Epub 2022 Nov 18. PMID: 36399291.
- Gall JE, Boyd RS, Rajakaruna N. Transfer of heavy metals through terrestrial food webs: a review. Environ Monit Assess. 2015 Apr;187(4):201. doi: 10.1007/s10661-015-4436-3. Epub 2015 Mar 24. PMID: 25800370.
- Briffa J, Sinagra E, Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020 Sep 8;6(9):e04691. doi: 10.1016/j.heliyon.2020.e04691. PMID: 32964150; PMCID: PMC7490536.
- Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front Pharmacol. 2021 Apr 13;12:643972. doi: 10.3389/fphar.2021.643972. PMID: 33927623; PMCID: PMC8078867.
- El Safty AMK. Heavy metal overload in autistic children. Egyptian Journal of Occupational Medicine. 2012; 36 (1): 97-106. DOI: 10.21608/ejom.2012.753
- Gamakaranage, C.: Heavy metals and autism. Journal of Heavy Metal Toxicity and Diseases. 2016; 1(3):12. ISSN 2473-6457.
- Jafari Mohammadabadi H, Rahmatian A, Sayehmiri F, Rafiei M. The Relationship Between the Level of Copper, Lead, Mercury and Autism Disorders: A Meta-Analysis. Pediatric Health Med Ther. 2020 Sep 21;11:369-378. doi: 10.2147/PHMT.S210042. PMID: 33061742; PMCID: PMC7519826.
- Gil-Hernández F, Gómez-Fernández AR, la Torre-Aguilar MJ, Pérez-Navero JL, Flores-Rojas K, Martín-Borreguero P, Gil-Campos M. Neurotoxicity by mercury is not associated with autism spectrum disorders in Spanish children. Ital J Pediatr. 2020 Feb 12;46(1):19. doi: 10.1186/s13052-020-0780-1. PMID: 32050998; PMCID: PMC7017444.
- Ogochukwu AO, Sha’ato R, Okeke F, Adekola OA, Agbele IE, Adegoke AO, Adeniyi AK. Evaluation of heavy metals profile in different brands of infant food nutrition. Chemical Science International Journal. 2019; 27(3):1-9. https://doi.org/10.9734/CSJI/2019/v27i330116.
- Zhang C, Gan C, Ding L, Xiong M, Zhang A, Li P. Maternal inorganic mercury exposure and renal effects in the Wanshan mercury mining area, southwest China. Ecotoxicol Environ Saf. 2020 Feb;189:109987. doi: 10.1016/j.ecoenv.2019.109987. Epub 2019 Nov 26. PMID: 31784104.
- Afonne OJ, Ifediba EC. Heavy Metals Risks in Plant Foods–Need to Step up Precautionary Measures. Curr. Opin. Toxicol. 2020; 22: 1–6. https://doi.org/10.1016/j.cotox.2019.12.006
- Frisbie SH, Mitchell EJ, Roudeau S, Domart F, Carmona A, Ortega R. Manganese levels in infant formula and young child nutritional beverages in the United States and France: Comparison to breast milk and regulations. PLoS One. 2019 Nov 5;14(11):e0223636. doi: 10.1371/journal.pone.0223636. PMID: 31689314; PMCID: PMC6830775.
- Flannery BM, Schaefer HR, Middleton KB. A scoping review of infant and children health effects associated with cadmium exposure. Regul Toxicol Pharmacol. 2022 Jun;131:105155. doi: 10.1016/j.yrtph.2022.105155. Epub 2022 Mar 4. PMID: 35257832.
- Technical SOP for Operation of Milestone UltraWAVE Microwave Digestion Unit El (n.d). https://dtsc.ca.gov/wp-content/uploads/sites/31/2020/06/03.3051.01_rev1_Berk_Technical-SOP-for-Operation-of-Milestone-UltraWAVE-Microwave-Digestion-Unit.pdf
- Weihang Y, Casey JF, Yongjum G. A new sample preparation method for crude or fuels oils by mineralization utilizing single reaction chamber microwave for broader multi-element analysis by ICP techniques. Fuel. 2017; 206: 64-79
- ASTDR. Minimal risk levels (MRLs) for Hazardous Substances. 2012. Minimal Risk Levels for Hazardous Substances | ATSDR (cdc.gov).
- Chuchu N, Patel B, Sebastian B, Exley C. The aluminium content of infant formulas remains too high. BMC Pediatr. 2013 Oct 8;13:162. doi: 10.1186/1471-2431-13-162. PMID: 24103160; PMCID: PMC3851493.
- Li J, Cao D, Huang Y, Chen B, Chen Z, Wang R, Dong Q, Wei Q, Liu L. Zinc Intakes and Health Outcomes: An Umbrella Review. Front Nutr. 2022 Feb 8;9:798078. doi: 10.3389/fnut.2022.798078. PMID: 35211497; PMCID: PMC8861317.
- Jana W, Lothan R. Handbook on the Toxicology of Metals. 2022.
- NIH. 2022. Zinc - Health Professional Fact Sheet (nih.gov)
- Hussain S, Khan M, Sheikh TMM, Mumtaz MZ, Chohan TA, Shamim S, Liu Y. Zinc Essentiality, Toxicity, and Its Bacterial Bioremediation: A Comprehensive Insight. Front Microbiol. 2022 May 31;13:900740. doi: 10.3389/fmicb.2022.900740. Erratum in: Front Microbiol. 2023 Jan 17;13:1133733. PMID: 35711754; PMCID: PMC9197589.
- Antonious GF, Dennis SO, Unrine JM, Snyder JC. Heavy metals uptake in plant parts of sweet potato grown in soil fertilized with municipal sewage sludge. International Journal of Geology. 2011; 1(5): 13-20.
- Narayan RR. Nickel essentiality, toxicity and the mechanism of toxicity in animals. Poll Res. 2020; 40(1):227-235. https://doi.org/10.1016/s1040-8428(01)00214-1
- Gonnelli C, Renella G. Chromium and Nickel. In: Alloway, B. (eds) Heavy Metals in Soils. Environmental Pollution. Dordrecht. 2013; 22. https://doi.org/10.1007/978-94-007-4470-7_11
- Nkwunonwo UC, Odika PO, Onyia NI. A Review of the Health Implications of Heavy Metals in Food Chain in Nigeria. ScientificWorldJournal. 2020 Apr 16;2020:6594109. doi: 10.1155/2020/6594109. PMID: 32351345; PMCID: PMC7182971.
- Cunningham E. What Role Does Diet Play in the Management of Nickel Allergy? J Acad Nutr Diet. 2017 Mar;117(3):500. doi: 10.1016/j.jand.2017.01.001. PMID: 28236963.
- Gu Z, de Silva S, Reichman SM. Arsenic Concentrations and Dietary Exposure in Rice-Based Infant Food in Australia. Int J Environ Res Public Health. 2020 Jan 8;17(2):415. doi: 10.3390/ijerph17020415. PMID: 31936289; PMCID: PMC7014030.
- Bair EC. A Narrative Review of Toxic Heavy Metal Content of Infant and Toddler Foods and Evaluation of United States Policy. Front Nutr. 2022 Jun 27;9:919913. doi: 10.3389/fnut.2022.919913. PMID: 35832055; PMCID: PMC9271943.
- Rafati Rahimzadeh M, Rafati Rahimzadeh M, Kazemi S, Moghadamnia AA. Cadmium toxicity and treatment: An update. Caspian J Intern Med. 2017 Summer;8(3):135-145. doi: 10.22088/cjim.8.3.135. PMID: 28932363; PMCID: PMC5596182.
- Alharbi NS, Akamsiei RM, Almaiman LA, Al-Samti MA, Al-Mutairi HS, Al-Owais BS, Alkhalaf MM, Bineid MA. Occurrence and dietary exposure assessment of heavy metals in baby foods in the Kingdom of Saudi Arabia. Food Sci Nutr. 2023 Jun 8;11(9):5270-5282. doi: 10.1002/fsn3.3485. PMID: 37701205; PMCID: PMC10494610.
- Morais S, Costa FG, Pereira M. Heavy metals and human health. Environmental health-emerging issues and practice. J.ed. Intech. 2012; 227-246. DOI: 10.5772/29869.
- Igweze ZN, Ekhator OC, Nwaogazie I, Orisakwe OE. Public Health and Paediatric Risk Assessment of Aluminium, Arsenic and Mercury in Infant Formulas Marketed in Nigeria. Sultan Qaboos Univ Med J. 2020 Feb;20(1):e63-e70. doi: 10.18295/squmj.2020.20.01.009. Epub 2020 Mar 9. PMID: 32190371; PMCID: PMC7065688.
- Munro C, Hlywka JJ, Kennepohl EM. Risk assessment of packaging materials. Food Addit Contam. 2002;19 Suppl:3-12. doi: 10.1080/02652030110102818. PMID: 11962713.
- ATSDR. Public Health Statement- Cadmium. 2012. https://www.atsdr.cdc.gov/phs/phs.asp?id=46&tid=15
- Polvara E, Spinazzè A, Invernizzi M, Cattaneo A, Sironi S, Cavallo DM. Toxicological assessment method for evaluating the occupational risk of dynamic olfactometry assessors. Regul Toxicol Pharmacol. 2021 Oct;125:105003. doi: 10.1016/j.yrtph.2021.105003. Epub 2021 Jul 12. PMID: 34265403.
- FDA. Closer to Zero Action Plan for Baby Foods. 2021. www.fda.gov/food/metals-and-your-food/closer-zero-action-plan-baby-foods
- Enrique M, Tatiana G. Analytical procedures for determining heavy metal contents in honey: A bioindicator of environmental pollution. INTECH. 2017; 14:311-324 DOI: 10.5772/66328.
- Muhammad S, Ali W, Ur Rehman I. Potentially Harmful Elements Accumulation and Health Risk Assessment of Edible Fish Tissues Caught from the Phander Valley, Northern Pakistan. Biol Trace Elem Res. 2022 Nov;200(11):4837-4845. doi: 10.1007/s12011-021-03051-z. Epub 2021 Dec 2. PMID: 34855146.
- Rai PK, Lee SS, Zhang M, Tsang YF, Kim KH. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ Int. 2019 Apr;125:365-385. doi: 10.1016/j.envint.2019.01.067. Epub 2019 Feb 8. PMID: 30743144.
- Nayak S, Sahu S, Patra S, John J. Assessment of Copper and Zinc Levels in Hair and Urine of Children With Attention Deficit Hyperactivity Disorder: A Case-Control Study in Eastern India. Cureus. 2021 Dec 25;13(12):e20692. doi: 10.7759/cureus.20692. PMID: 35106229; PMCID: PMC8786440.
- Zaky EA. Toxic heavy metals and autism spectrum disorder: is there a link? Journal of child and adolescent behavior. 2017; 5:2. http://dx.doi.org/10.4172/2375-4494.1000336
- Zhang W, Liu Y, Liu Y, Liang B, Zhou H, Li Y, Zhang Y, Huang J, Yu C, Chen K. An Assessment of Dietary Exposure to Cadmium in Residents of Guangzhou, China. Int J Environ Res Public Health. 2018 Mar 20;15(3):556. doi: 10.3390/ijerph15030556. PMID: 29558399; PMCID: PMC5877101.